50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 1 of 35
Table of Contents
The purpose of this training is to assist Public Health Providers and CDC personnel in understanding the new CDC Specimen Submission form for
specimens of Food, Environmental, Medical Device, or Biologic (FEMB) origin. The training is helpful to those responsible for preparing CDC
Specimen Submission forms for specimens submitted to the CDC for testing.
The training is organized by the following sections and supporting topics:
Overview
Webinar Objectives
Changes to the Overall Process
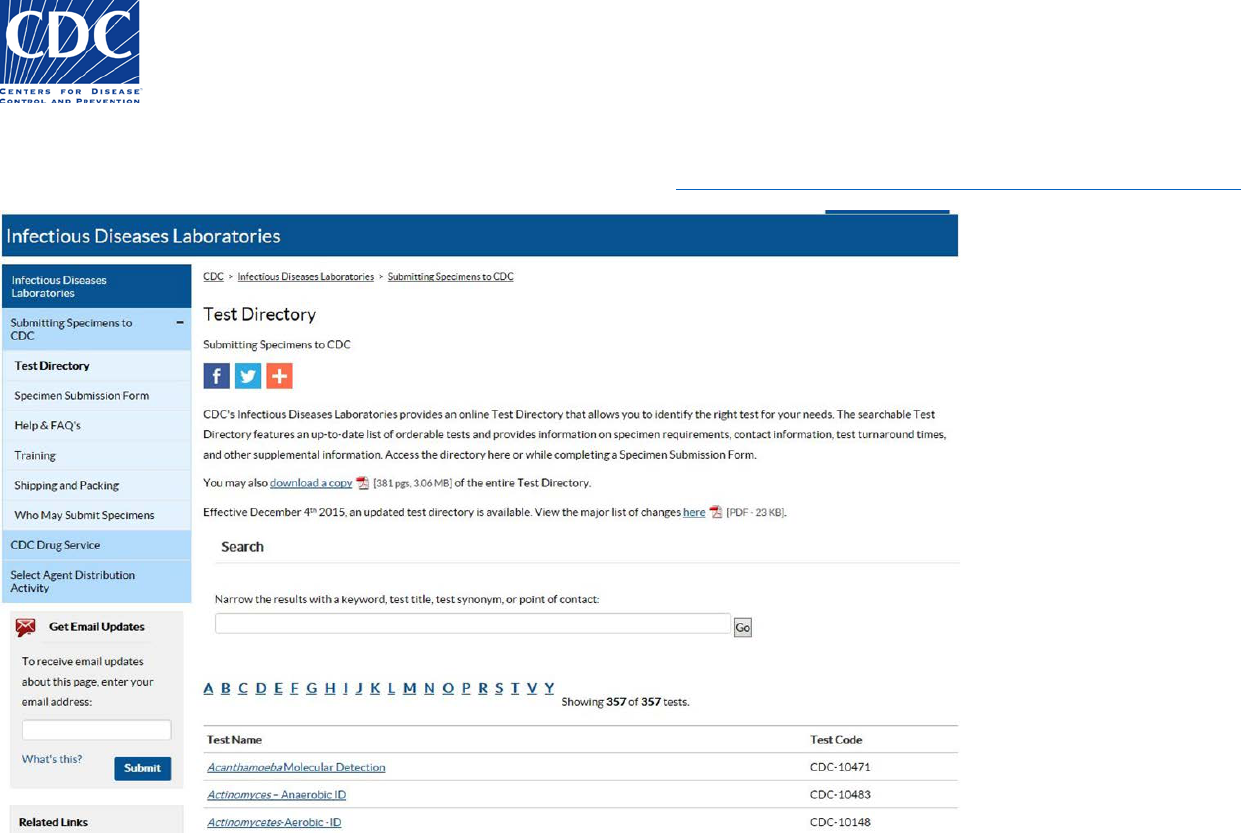
CDC Infectious Diseases Test Directory
Benefits to PHLs
What is the Specimen Submission Form?
How the Form is Organized
Entering Data
Using Pick-lists
Entering Dates
Entering Test Order Name
Test Order Requirements
― Prior Approval
― Supplemental Form
― Entering Submitter Data
― Entering Email Address
Sections on the Form
Origin
Laboratory Examination Requested
Specimen Information
CDC Use Only
State PHL Submitter
Original Submitter
Intermediate Submitter
Specimen Identifiers
Sample Information
Sample Location Information
Additional Sample Information
Previous Laboratory Results
Comments
CDC Use Only Barcodes
Expiring Template Forms
How to Obtain a Current Template Form

50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 2 of 35
Overview
Training Objectives
Upon completion of this material, users will be able to:
List the overall improvements CDC ID laboratories are making to the specimen accessioning and reporting process
Enumerate the benefits of using the new electronic Adobe specimen submission form
Enter the necessary information on the updated submission form
Link to the CDC Test Directory for essential information when ordering a test
Changes to the overall process
Changes to the overall process include:
The 3
rd
barcode encodes page 2 of the Specimen Submission form, not the Intermediate Submitter information. Therefore the
Intermediate Submitter information will be manually uploaded by CDC recipients.
Submitter information is a dropdown menu selection.
The “State PHL…”, “Original Submitter”, and “Intermediate Submitter” sections now contain a direct phone number and email address
for the Point of Contact, and no longer contain a phone number for the institution.
Updated Test Directory of Services
Enhancements that were implemented for Form 50.34 Version 2.0:
Federal, State, and International Submitters can now use the dropdown feature for Institution Name, allowing users to select their
Institution. Institutions are listed in alphabetically and users can easily find their Institution by typing the first letter of their state.
Selecting Institution Name from the drop-down menu causes the Address, Fax, and Institutional Email fields to be auto-populated with
contact information from standardized submitter records.
The ‘Previous Laboratory Results/Comments’ is now two separate fields, “Previous Laboratory Results” and “Comments”
When a test order with additional information or pre-approval requirement is selected, an information icon or
with alert pop-up window occurs.
“Specimen Source (Type)” is a required field and will be highlighted in red when not data is not entered.
Version and expiration date are updated: CDC 50.34 v2.0 (Expires December 8, 2017 at 11:59pm).

50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 5 of 35
Benefits to PHLs
The benefits to the PHLs are included below:
Select a test offered by CDC via dropdown menu
Select their Institution Name via dropdown menu and have contact information auto-populated with standardized record information
Ability to electronically enter data into the form and save it
Control the distribution of the new form with their clinical labs
Increase accuracy of information entered into the CDC Laboratory Information Management System (LIMS)
Delivery of results faster as encrypted PDFs sent by secure email
Prepare for electronic messaging
Links automatically to supplemental forms, additional information, and CDC contacts for pre-approval and consultation.
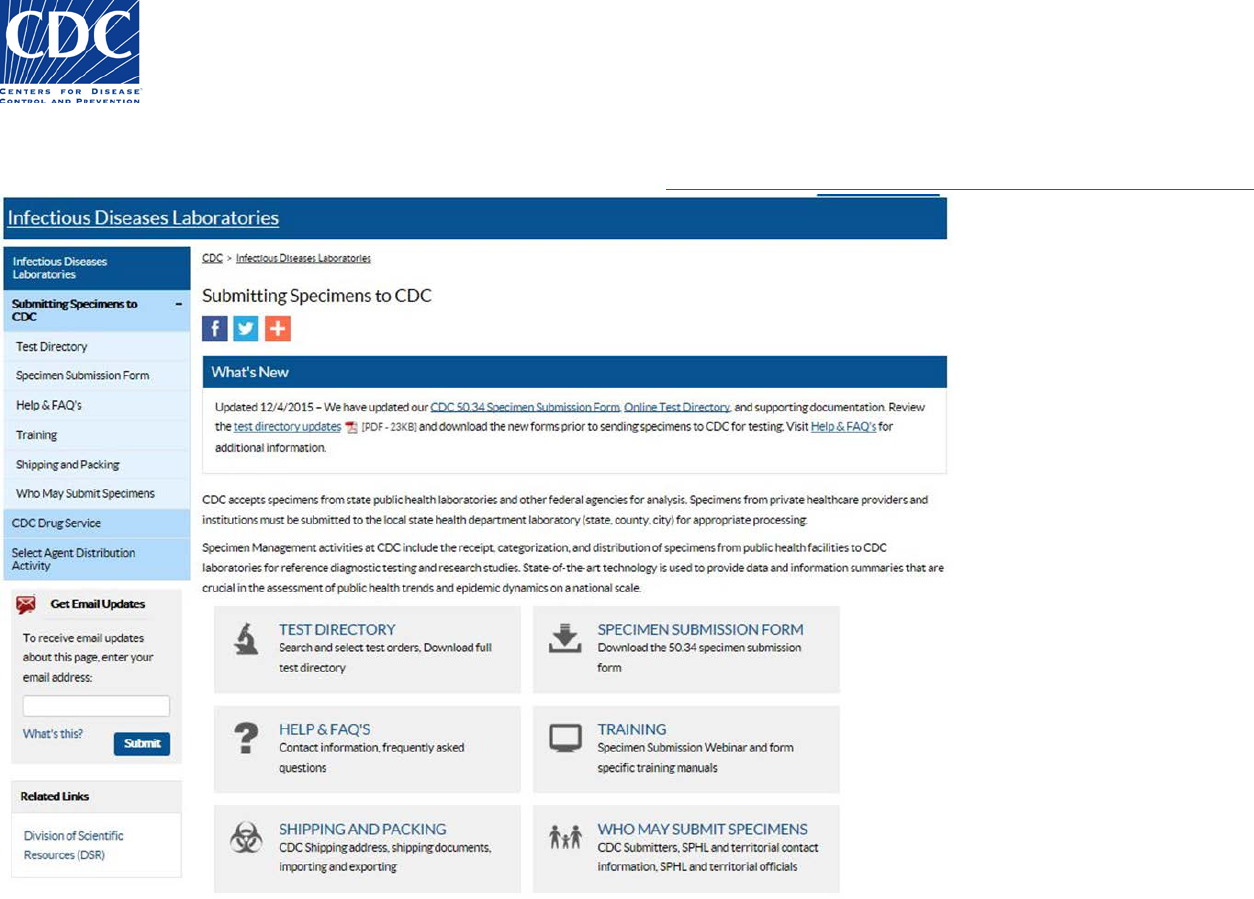
What is the Specimen Submission Form?
Public Health Providers and other Submitters must complete a Specimen Submission form for each specimen they submit to the Centers for
Disease Control and Prevention (CDC) Infectious Diseases Laboratories for testing. The new CDC 50.34 Specimen Submission form provides the
most effective way to record the necessary information required to identify the specimen and submitter.
The Specimen Submission form provides the following benefits:
The form is downloadable and the data you enter can be saved to the form at any time.
The form can be filled out on your computer, printed, and then sent to the CDC with the specimen. This ensures the content is legible
which reduces the possibility of erroneous data.
Printing is prevented until all required data fields are filled appropriately to prevent missing information being sent to CDC.
Pick-lists are provided to allow for the selection of valid field values which ensures the integrity of the data.
Some pick-lists auto-populate fields which saves submitters time
Barcodes expedite the process of transferring data from the form into the CDC Laboratory Information Management System (LIMS),
which eliminates the need for manual entry and reduces the amount of human error.
This document will provide training by introducing you to the CDC Specimen Submission form and provide instructions on how to fill out the
form.

50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 6 of 35
How the Form is Organized
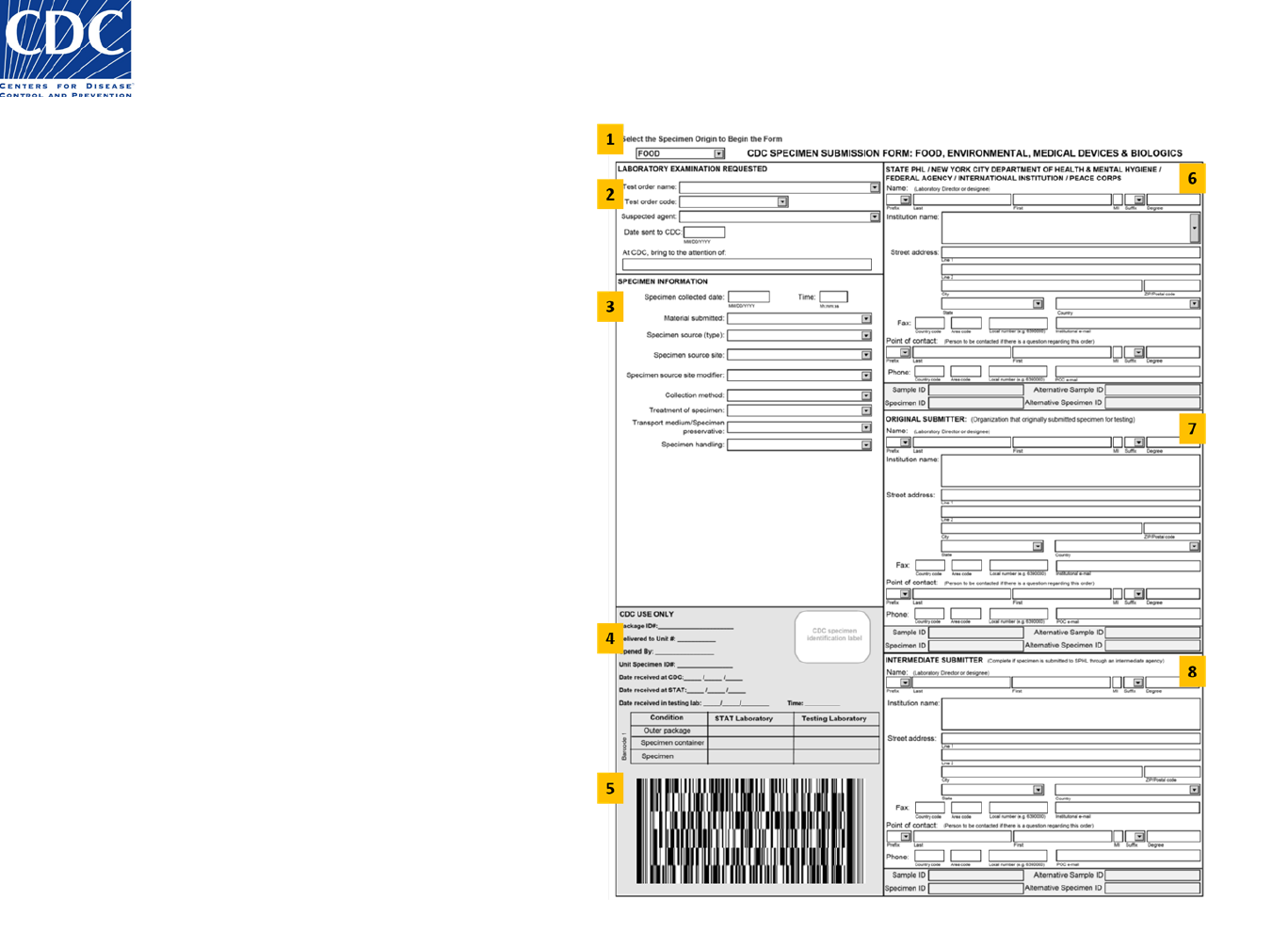
The FEMB Specimen Submission form is a two-sided document that is divided into 16 sections.
Notes:
• The section numbers in the list below correspond to the section numbers on the sample form in figures 1 and 2.
• The barcodes on both sides of the form will not appear until the form is validated and printed successfully.
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 7 of 35
Front of the Form (Figure 1)
1. Origin
2. Laboratory Examination Requested
3. Specimen Information
4. CDC Use Only
5. Barcode 1
6. State PHL Submitter
7. Original Submitter
8. Intermediate Submitter
Figure 1: Specimen Submission Form (Front)
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 8 of 35
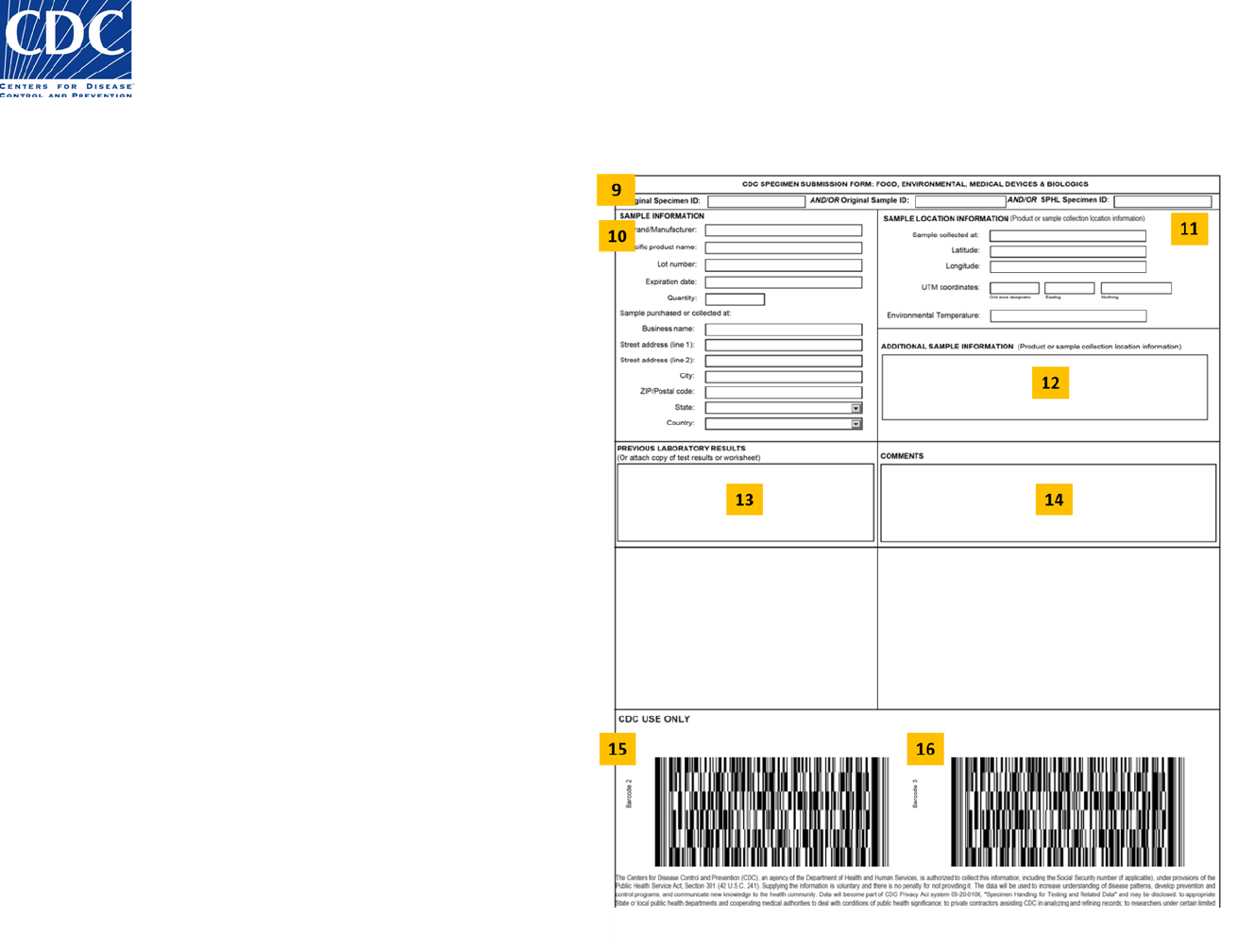
Back of the Form (Figure 2)
9. Specimen Identifiers
10. Sample Information
11. Sample Location Information
12. Additional Sample Information
13. Previous Laboratory Results
14. Comments
15. Barcode 2
16. Barcode 3
Figure 3: Specimen Submission Form (Back)
Figure 2: Specimen Submission Form (Back)

50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 9 of 35
Entering Data
Before we take a look at the individual sections in detail, we will show you how to enter and correct data on the form
Using Picklists
Overview
Pick-lists are available for many fields to provide you with
a convenient way to select field values. Click the down-
arrow and the pick-list appears, or type the first letter of
the value you want to jump to the selection.
If your information is not in the pick-list, select the blank
field and hand-write your information after you print the
form. Some fields with pick-lists e.g. sex, may not have a
blank row at the top. In these instances, you must select
from a value in that pick-list; values may not be hand-
entered.
Action
Follow these steps to select a value from a pick-list:
Click the down-arrow for the field. The pick-list for the
field appears.
Click the value on the pick-list which best represents your
selection.
Result
The value that you selected appears in the field.
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 10 of 35
Entering Dates
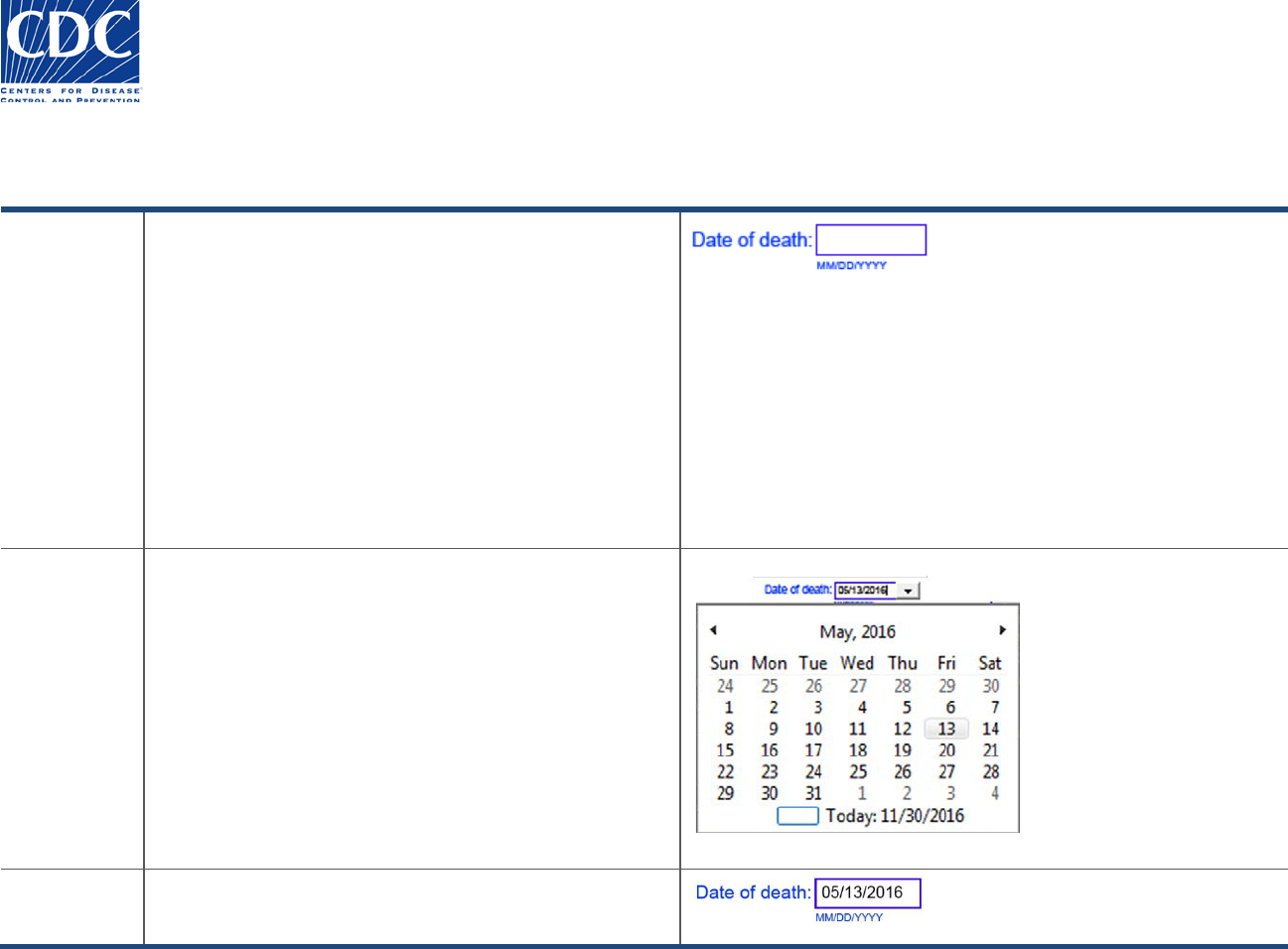
Overview
Dates may be entered in one of two ways, you may enter
the date in “MM/DD/YYYY” format, or you may select the
date from the calendar.
When you enter an invalid date, or the date you enter
does not meet the rules established for that date, you will
receive an error message. Some examples include:
Date of death cannot be after today’s date.
Start Date cannot be after End Date.
Invalid date format. Please enter date as
“MM/DD/YYYY”.
Action
You may enter a date using the format: MM/DD/YYYY, or
follow these steps to select the date from the calendar:
1. Click inside the date field. The drop-down arrow
appears.
2. Click the drop-down arrow. The calendar appears.
3. Select a specific day using the calendar format, or
click the blue-lined box at the bottom of the calendar
to select today’s date.
Note: Make sure you are in the correct calendar month
and year.
Result
The date you entered or selected appears in the date
field.
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 11 of 35
Entering Test Order Name
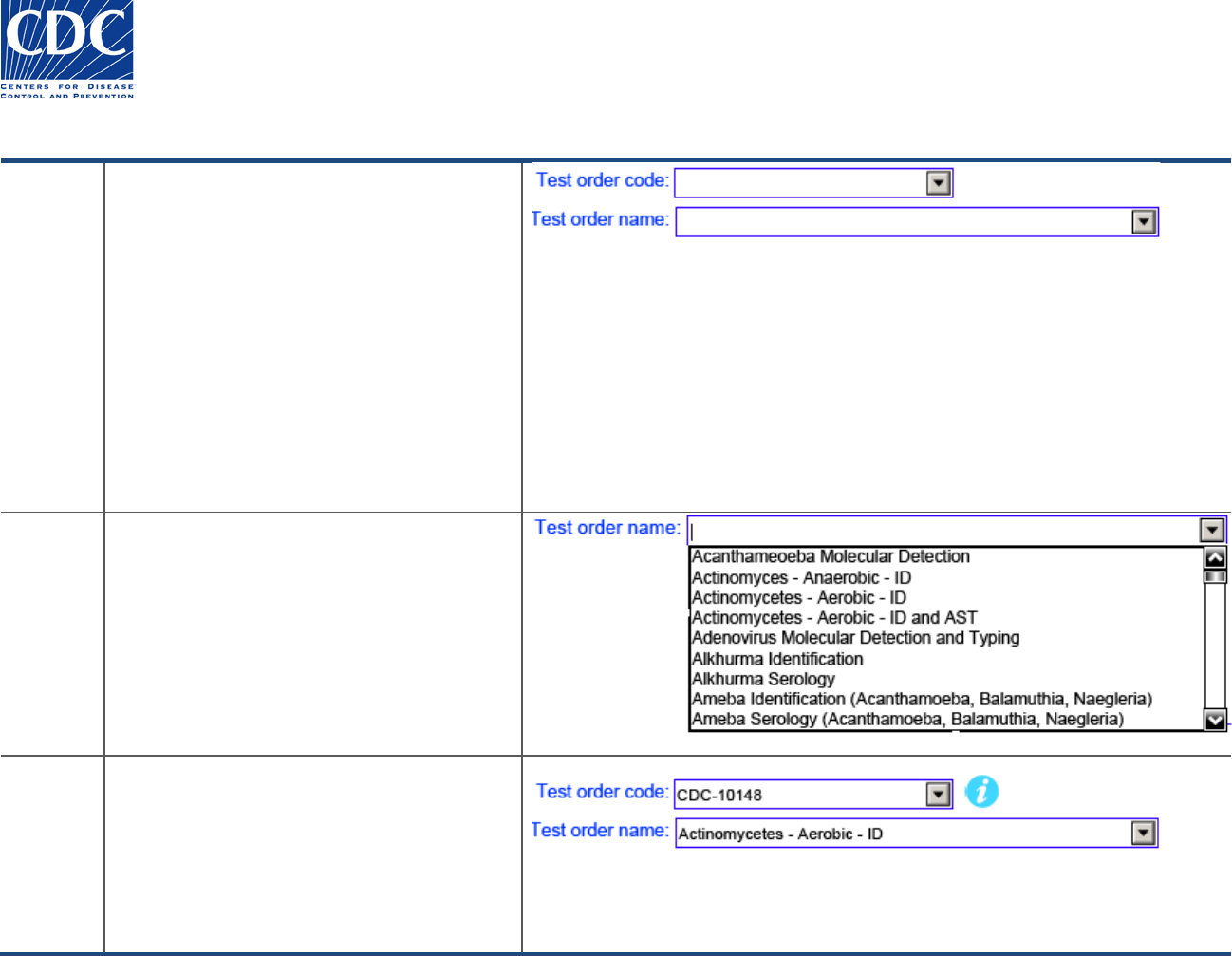
Overview
The test order name is mandatory in order to
submit specimens to the CDC for testing. The
test order code will auto-populate based on
the test order name selected. Alternatively, if
you know the test order code, select it, and
the test order name will auto-populate.
If the test order code and name are left blank
and you try to print the form, you will receive
the following error message:
“The following required fields are empty:
Required field – Test order name”
Action
Follow these steps to select the test order
name:
1. Click the Test Order Name drop-down
arrow. The pick-list appears.
2. Select the Test Order Name from the pick-
list.
Result
The Test Order Name you selected appears,
and the Test Order Code auto-populates
based on your selection.
The Information icon appears next to the Test
Order Code. Click this icon to find additional
information for the specific test order.
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 12 of 35
Test Order Name Requirements – Prior Approval and Supplemental Forms
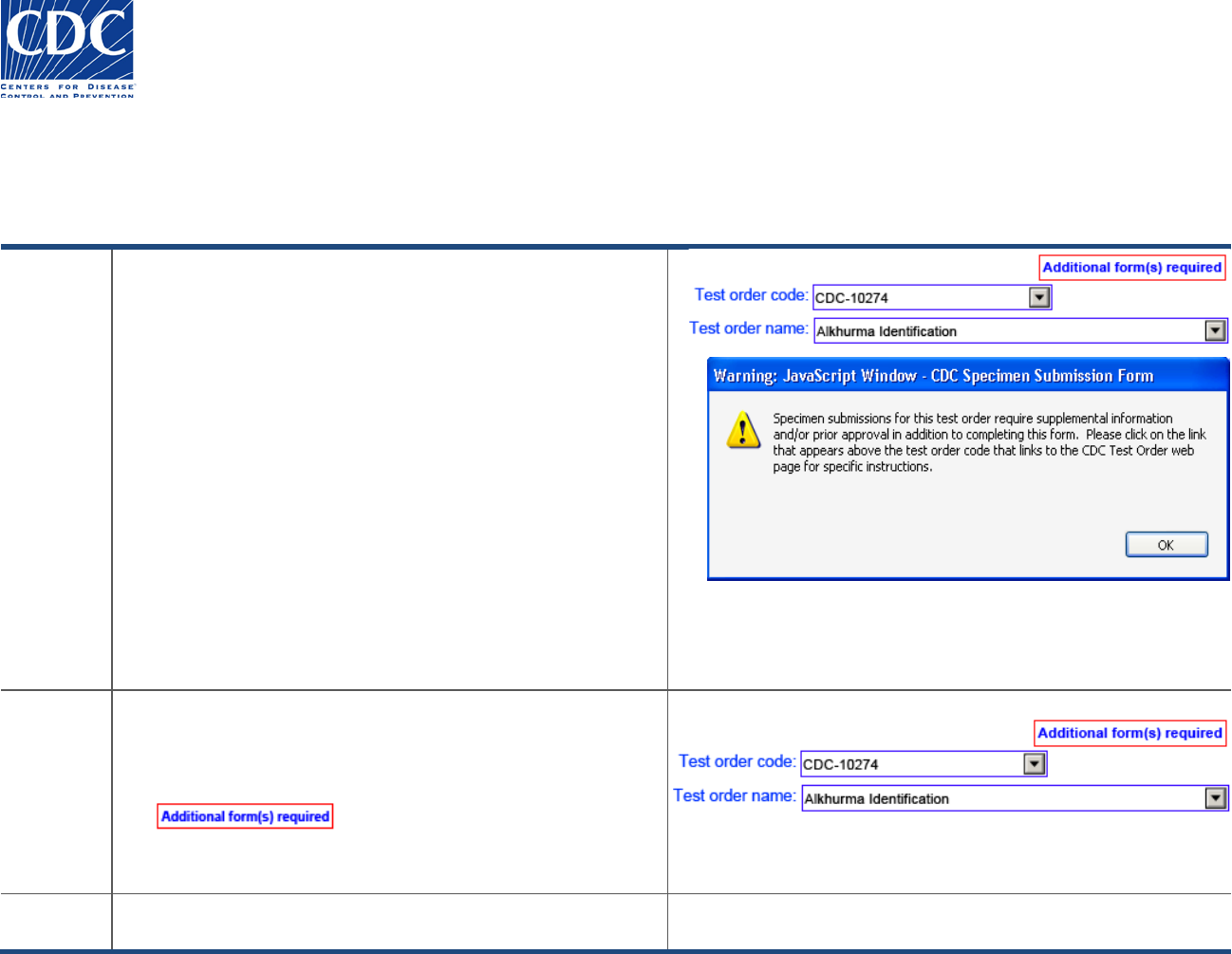
Overview
The test order name is mandatory. The test order code will
auto-populate based on the test order name selected.
Alternatively, if you know the test order code, select it, and
the test order name will auto-populate. If you select a test
order code, please verify that the test order name matches the
test order you wish to order.
For some test orders, you will be required to provide
supplemental information. For instance, in this example, you
are required to fill out an additional form for test order code,
‘CDC-10274’. After selecting the test order code, you will
receive the following message:
“Specimen submissions for this test order require supplemental
information and/or prior approval in addition to completing
this form. Please click on the link that appears above the test
order code that links to the CDC Test Order web page for
specific instructions.
Action
Follow these steps to locate the additional required form:
1. Click OK to acknowledge the warning message.
The Help icon next to the test order code disappears and is
replaced by the “Additional form(s) required” button:
2. Click the “Additional form(s) required” button to access prior
approval or supplemental form instructions.
Result
The CDC Test Order web page appears with specific
instructions for prior approval or supplemental forms.
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 13 of 35
Entering Submitter Data
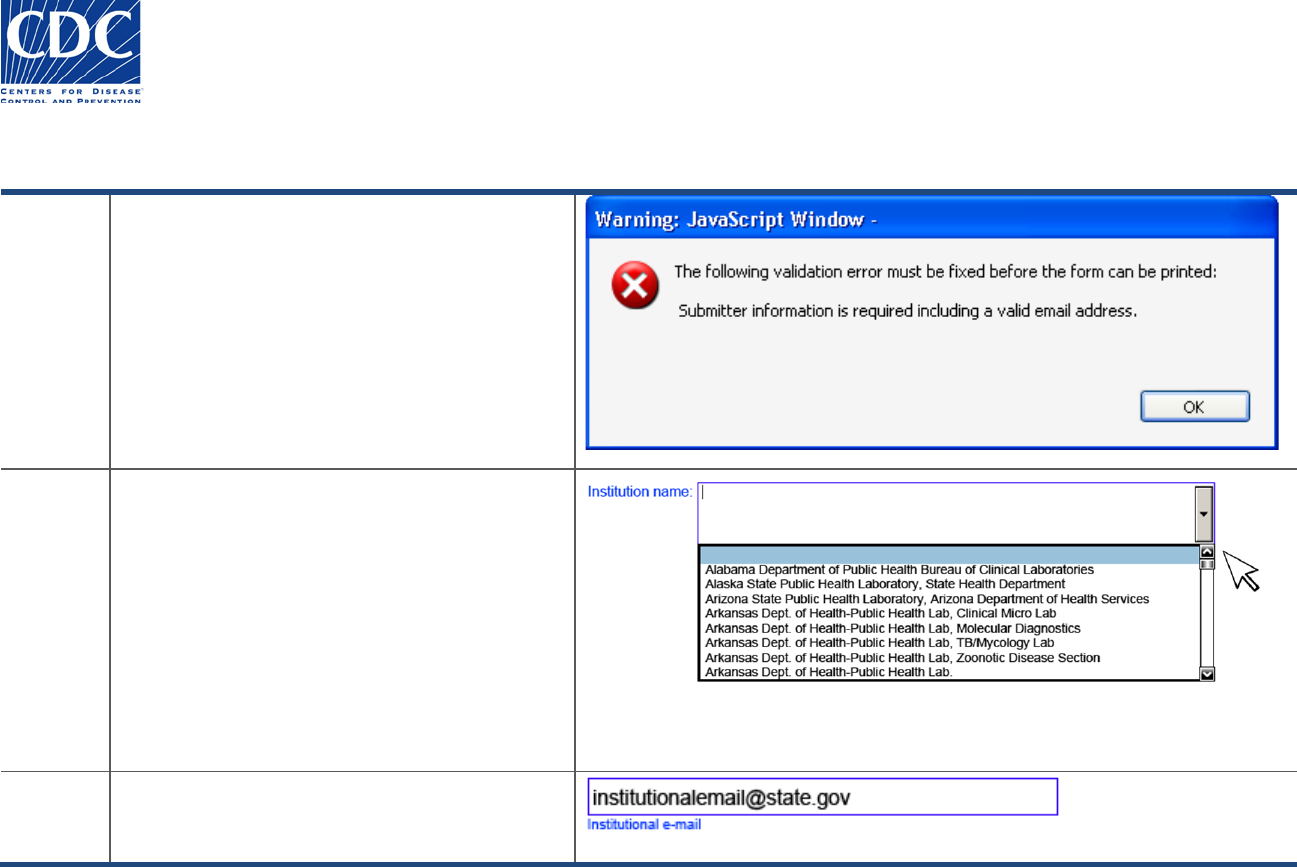
Overview
You must enter data in the State PHL Submitter
section, including a standard address and valid
email address.
If you leave the submitter data blank, the
following message appears:
“Submitter information is required including a
valid email address”.
Action
Follow these steps to add submitter data:
1. Click OK to acknowledge the warning message.
2. Under the “State PHL…” section, select your
institution from the “Institution Name” dropdow
n
menu. You can also type the first letter of your
state to find your institution name more quickly.
Contact information from standardized records
will appear.
3. If the institutional information that appears is not
correct, erase the Institution Name and enter all
data fields manually.
Result
The submitter data and email address appears.
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 14 of 35
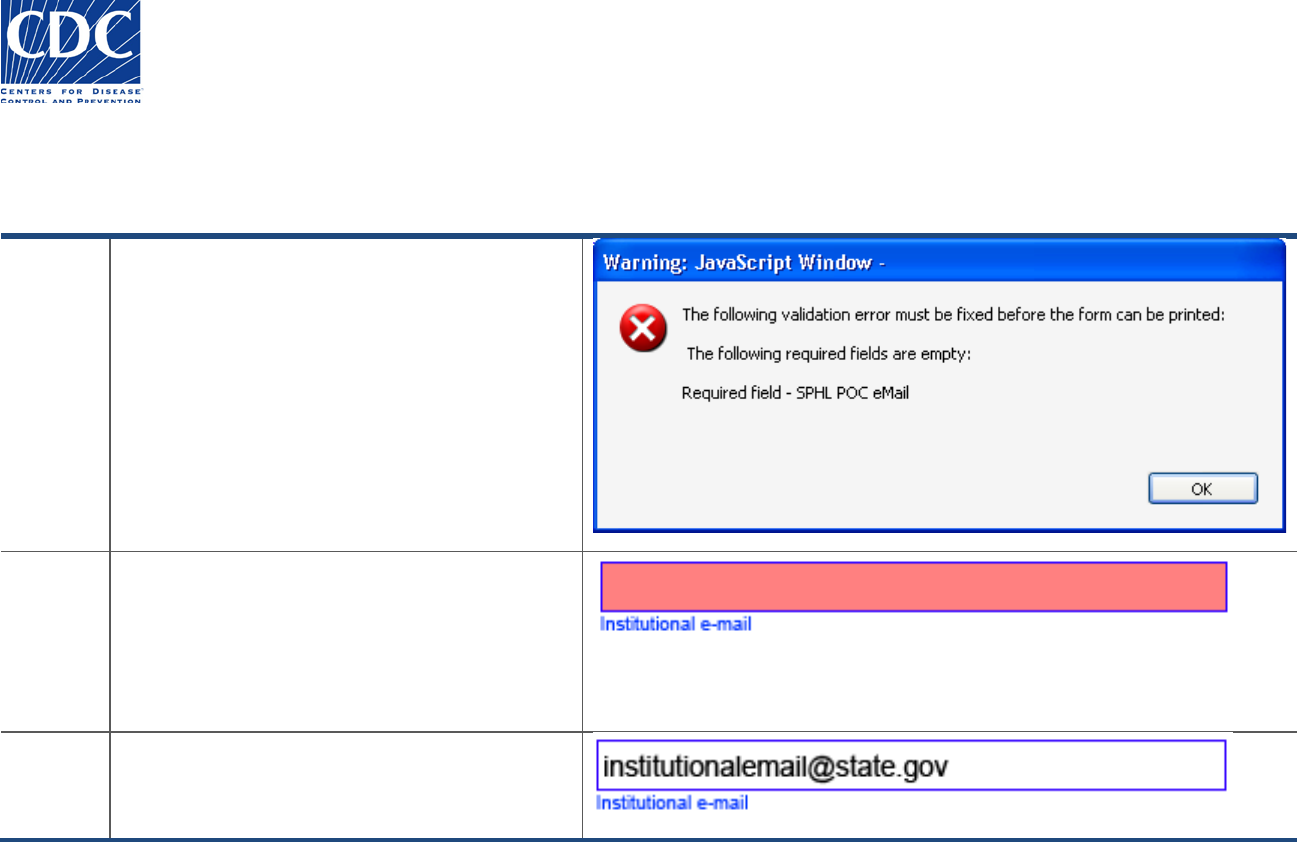
Entering Email Address
Overview
If you do not enter an email address, the
following warning message appears:
“The following required fields are empty: Required
field – SPHL approved laboratory email address”.
If the institution does not have an approved
laboratory email address, then enter the Lab
Director’s email address.
Action
Follow these steps to correct the email address:
1. Click OK to acknowledge the warning message.
The erroneous email address field appears
highlighted in red.
2. Enter the email address in the following format:
name@somewhere.com.
Result
The corrected email address appears.

50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 15 of 35
Sections of the Form
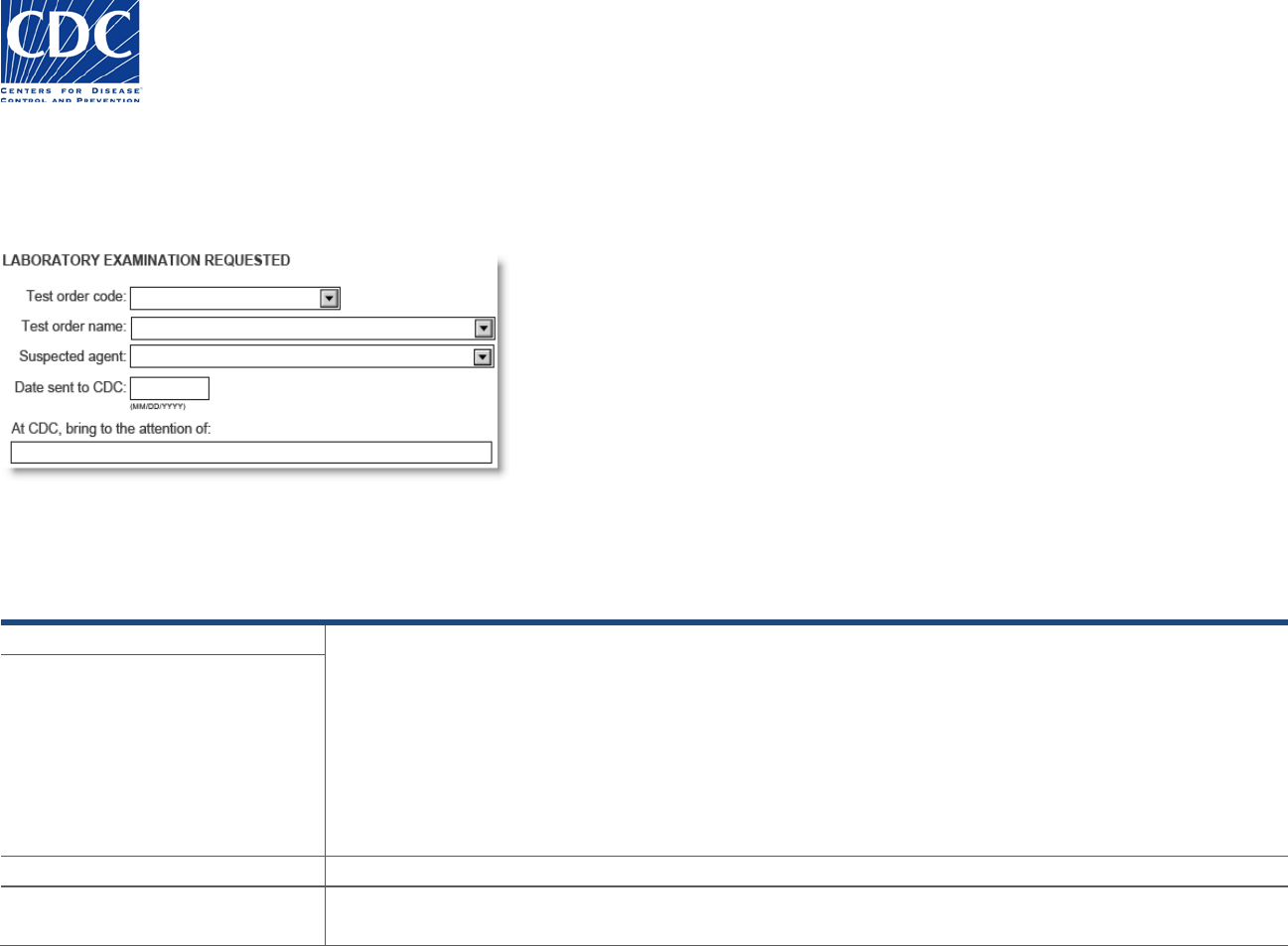
Origin
The Origin section appears below:
Select the Specimen Origin to Begin the Form
This section is used to specify the origin for the material you are submitting. The fields on the form will change based on the origin selected.
There will be three distinctly different forms in this form. Fields on the Human form are different from those on the Animal form and these are
different from a single form that is used for submitting specimens of Food, Environmental, Medical Device, or Biologic origin.
Field Name
Field Instructions
Origin
Select the origin for the material you are submitting from the pick-list.
Valid options are:
Human
Animal
Food
Environmental
Medical Device
Biologic
The form will populate the fields that are specific to the origin selected.
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 16 of 35
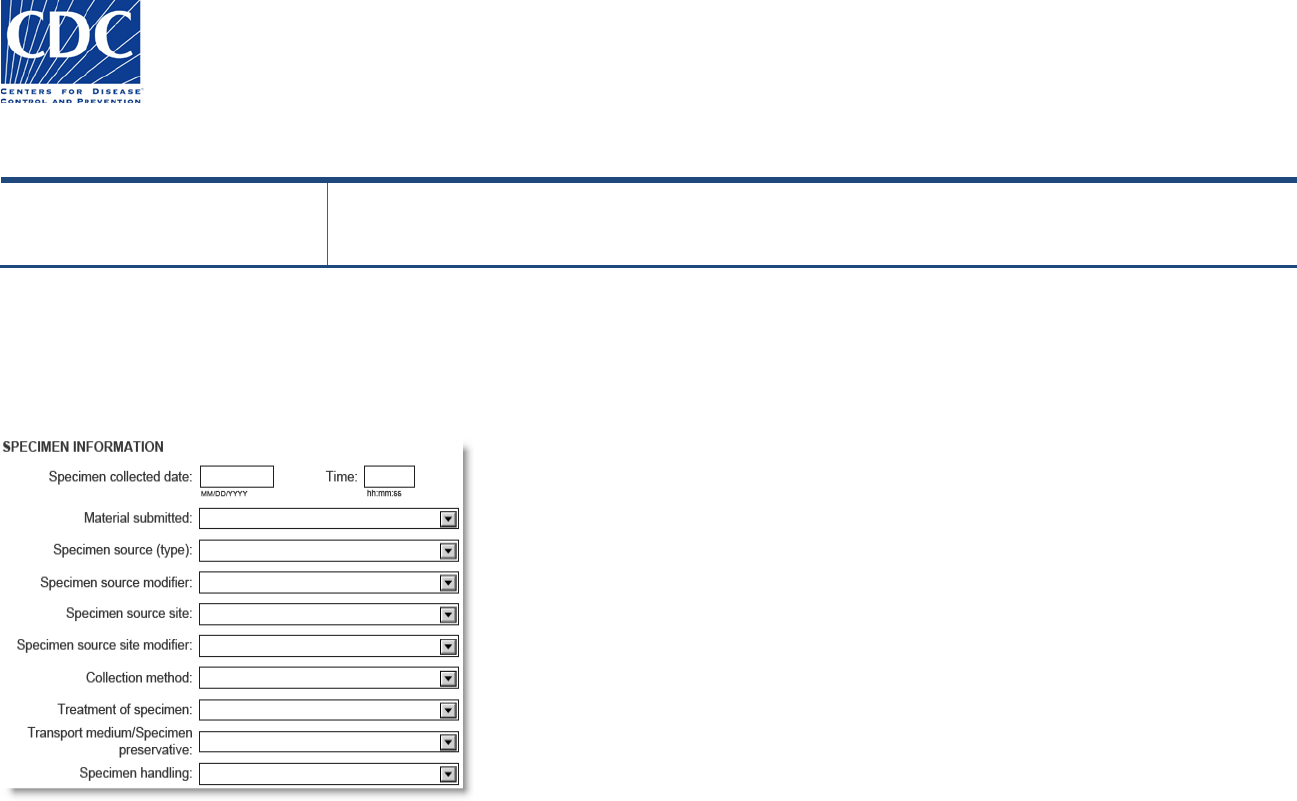
Laboratory Examination Requested
Th
e Laboratory Examination Requested section appears below:
Th
is section is used to specify the test order name and code assigned to the specimen, the suspected agent, the date the specimen was sent to
the CDC, and to whom the specimen was sent. Valid field values may be selected from the pick-lists, where available.
Field Name
Field Instructions
Test Order Code
The test order name is mandatory. The test order code will auto-populate based on the test order name
selected. Alternatively, if you know the test order code, select it, and the test order name will auto-
populate.
In some cases, you may receive the following message:
“Specimen submissions for this test order require supplemental information and/or prior approval in
addition to completing this form. Please click on the link that appears above the test order code that links
to the CDC Test Order web page for specific instructions.”
In this case, click the link that appears and follow the instructions.
Test Order Name
Suspected Agent
Select the suspected agent from the list of bacteria, viruses, fungi, and parasites.
Date Sent to CDC
Enter/select the date the specimen was shipped to the CDC. This date is important because it lets us
know if the specimen is delayed in transit and whether the delay affects its suitability for testing.
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 17 of 35
Field Name
Field Instructions
At CDC, bring to attention of:
If you have prior approval or have talked with someone in the CDC laboratory about this specimen/order,
enter the name of that person to facilitate the testing. This space may be left blank if prior approval for
testing is not required.
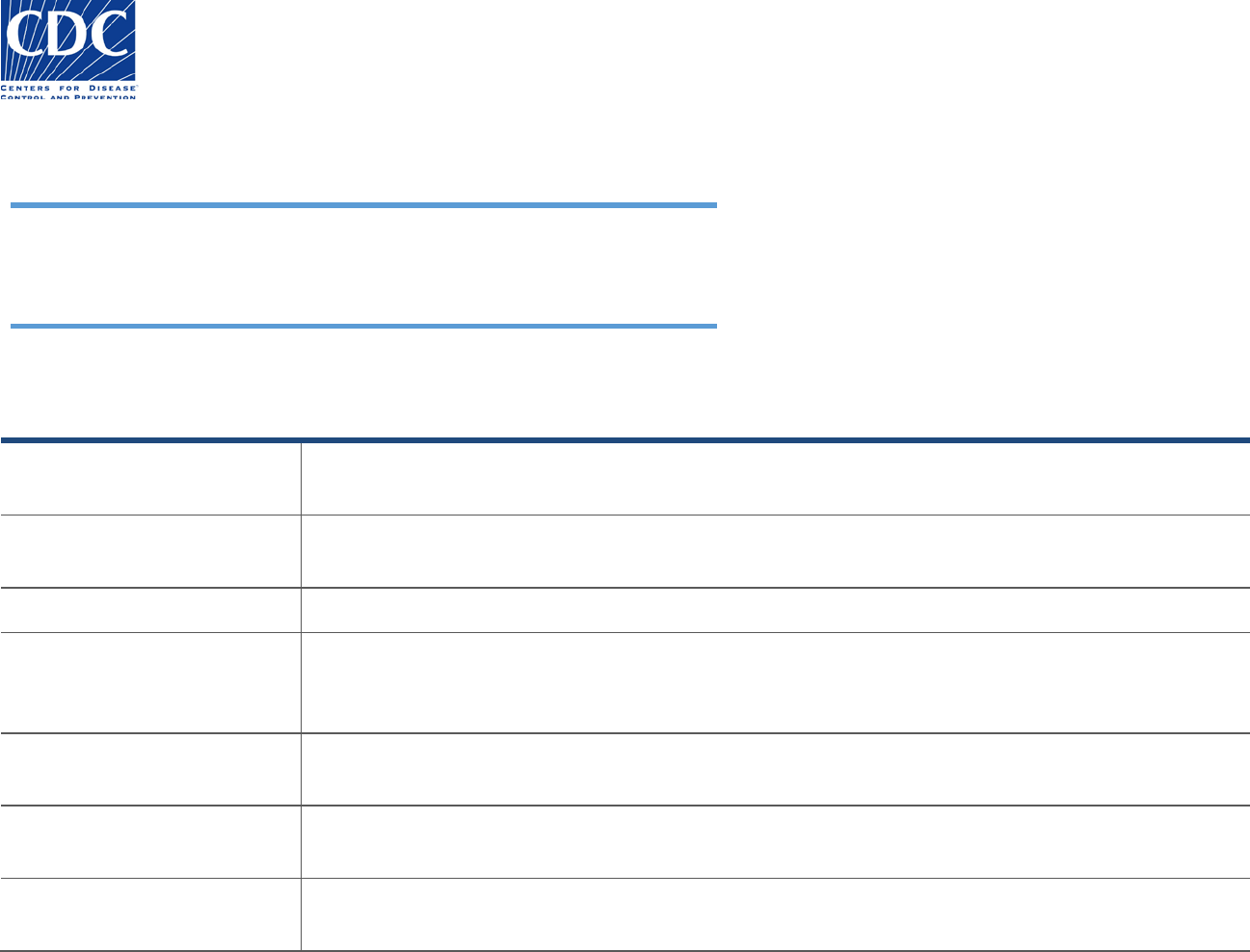
Specimen Information
The Specimen Information section appears below:
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 18 of 35
This section is used to enter pertinent information about the specimen that will allow the testing laboratory to determine the suitability for
testing.
Field Name
Field Instructions
Specimen collected (Date, Time)
Enter the date the specimen was collected as MM/DD/YYYY. Enter the time as HH:MM:SS. If a date is
entered and the time is left blank, the default time is 01:00:00. Blank minutes or seconds default to 00.
Material submitted
Select the original specimen or a specimen derivative such as an isolate or nucleic acid that has been
extracted from the original specimen.
Specimen Source (Type)
Select the type of specimen that was collected, or the specimen where the isolate was recovered.
Specimen Source Modifier
Used to indicate the status of a serum specimen, i.e., whether it was collected from a sample during the
'acute' or 'convalescent' phase of an infection. Other values such as S1 are intended for specimens being
collected for studies.
Anatomic (body) site
Select the anatomic (body) site from which the original specimen was taken (e.g., arm, leg, liver). In most
cases, this field will not be filled for specimens such as blood.
Anatomic (body) site modifier
Provides more information about the anatomic (body) site from which the specimen was taken such as
'right' (arm), if applicable. Not required for blood or serum.
Collection method
Provides information about how the specimen was collected. This is critical information about the
adequacy of the specimen collected, and includes values such as 'Aspiration' and ‘Biopsy’.
Note: Valid values for all fields are available in the pick-lists. If the value you
require is not in a pick-list, select the blank entry, and then handwrite the value
on the printed form.

50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 19 of 35
Field Name
Field Instructions
Treatment of specimen
Select what treatment the specimen has received (e.g., Centrifugation).
Transport medium/Specimen
preservative
Select the medium in which the specimen was submitted, or the substance that has been added to the
specimen, to ensure its suitability for testing (e.g., Campy-BAP agar).
Specimen handling
Select the temperature or other conditions under which you are submitting the specimen (e.g., dry ice,
ambient temperature).

50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 20 of 35
CDC Use Only
The CDC Use Only section appears below:
This section is reserved for CDC use only. The CDC personnel
responsible for processing the specimen package will use this section
to record the package identifiers, dates of receipt, and the condition
of the package and contents.
Field Name
Field Instructions
Package ID#
CDC use only
Delivered to Unit#
CDC use only
Unit Specimen ID#
CDC use only
Date received at CDC
CDC use only
Date received in testing Lab
CDC use only
Time received in testing Lab
CDC use only
Condition Outer Package
CDC use only
Condition Specimen Container
CDC use only
Condition Specimen
CDC use only
Note: The fields in this section are protected. The information
must be hand written directly on the paper form by the
appropriate CDC personnel.

50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 21 of 35
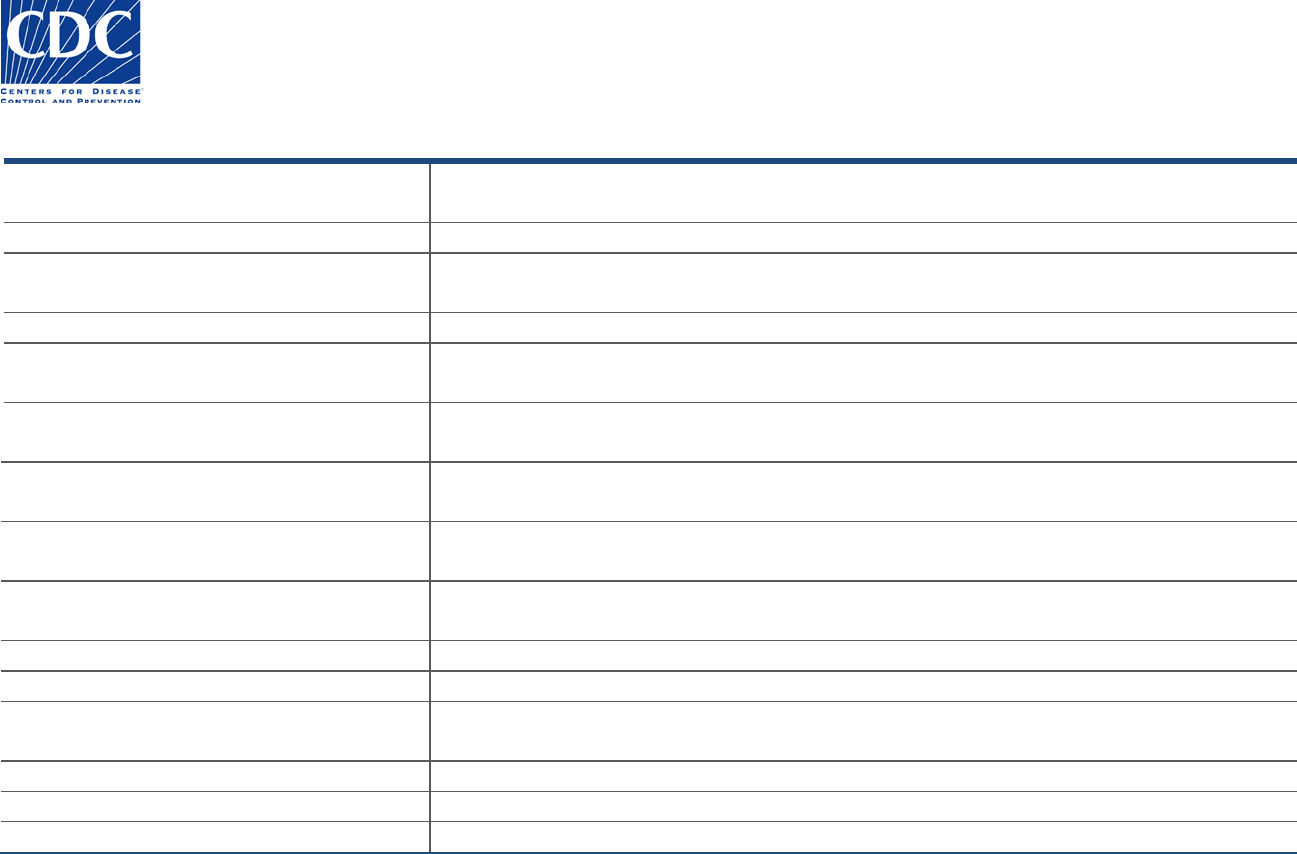
State PHL Submitter
The State PHL Submitter section appears below:
This section includes the submitter information for the State PHL, New York City HD laboratory, Federal Agency, International Institution, and
Peace Corps that submitted the specimen for examination.
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 22 of 35
Field Name
Field Instructions
Name (last, first, MI, suffix, degree)
Name of person authorizing reference testing from the CDC. This person is usually the
laboratory director or their designee.
Institution Name
Use the dropdown menu to select the institution name and specific department, if available.
Street address 1
Will autofill if dropdown is used or enter the street address, including the specific floor/room
number.
Street address 2
Will autofill if dropdown is used or enter the post office box or mailstop.
City, State, Zipcode, Country
Will autofill if dropdown is used or enter the city, state or province, zip or postal code, and
country.
Phone (country code, area code, local
number, extension)
Will autofill if dropdown is used or enter local phone number for the laboratory, including
country code and area code (numbers only; no spaces or special characters).
Fax (country, area code, local number)
Will autofill if dropdown is used or enter country code, area code, and local number in the
appropriate fields (numbers only; no spaces or special characters).
Institutional e-mail
Will autofill if dropdown is used or enter a standardized institution or lab email address that is
approved for the CDC form.
Point of Contact (prefix, last, first, middle
initial, suffix, degree)
Enter the primary or alternative person in the laboratory who can answer questions regarding
the specimen submission.
Phone (country, area code, local number)
Enter the Point of Contact’s direct phone number
POC e-mail
Enter the Point of Contact’s direct email address
Sample ID
Enter the primary sample ID if assigned by the State PHL (SPHL). The number might be used
for studies.
Specimen ID
Enter the primary specimen ID if assigned by the SPHL. The number might be used for studies.
Alternative Sample ID
Alternative sample ID if assigned by the SPHL.
Alternative Specimen ID
Alternative specimen ID if assigned by the SPHL.

50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 23 of 35
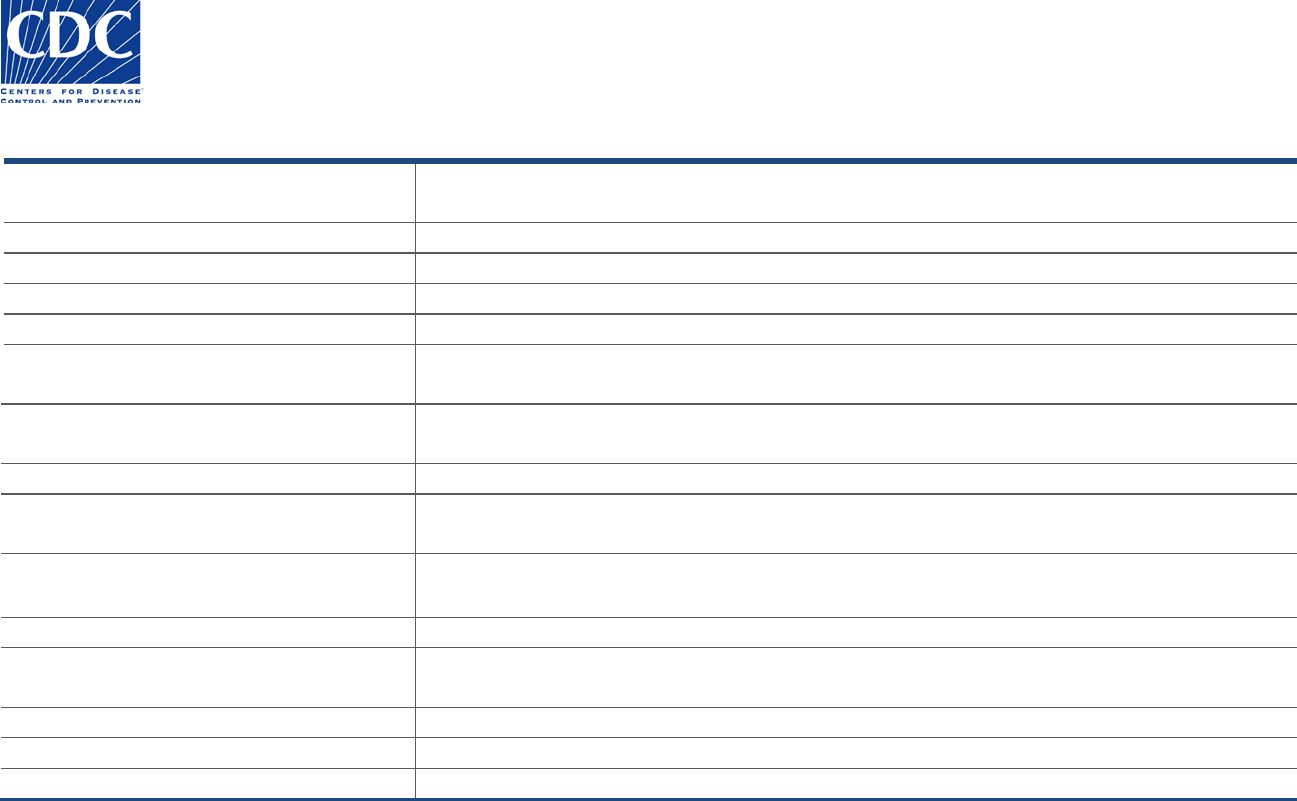
Original Submitter
The Original Submitter section appears below:
This section includes the submitter information for the laboratory, hospital, or clinic that originally submitted the specimen for examination.
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 24 of 35
Field Name
Field Instructions
Name (last, first, MI, suffix, degree)
Name of person authorizing reference testing to be performed. This person is usually the
laboratory director or their designee.
Institution Name
Enter the institution name and specific department
Street address 1
Enter the street address, including the specific floor/room
Street address 2
Enter the post office box or mailstop.
City, State, Zipcode, Country
Enter the city, state or province, zip or postal code, and country.
Phone (country code, area code, local
number, extension)
Enter local phone number for the laboratory, including country code and area code (numbers
only; no spaces or special characters).
Fax (country code, area code, local number)
Enter country code, area code, and local number in the appropriate fields (numbers only; no
spaces or special characters).
Institutional e-mail
Enter an email address for the institution or lab director.
Point of Contact (prefix, last, first, middle
initial, suffix, degree)
Enter the primary person in the laboratory who can answer questions regarding the specimen
submission.
Phone (country, area code, local number)
Enter the Point of Contact’s direct phone number
POC e-mail
Enter the Point of Contact’s direct email address
Sample ID
Enter the primary sample ID if assigned by the State PHL (SPHL). The number might be used for
studies.
Specimen ID
Enter the primary specimen ID if assigned by the SPHL. The number might be used for studies.
Alternative Sample ID
Alternative sample ID if assigned by the SPHL.
Alternative Specimen ID
Alternative specimen ID if assigned by the SPHL.

50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 25 of 35
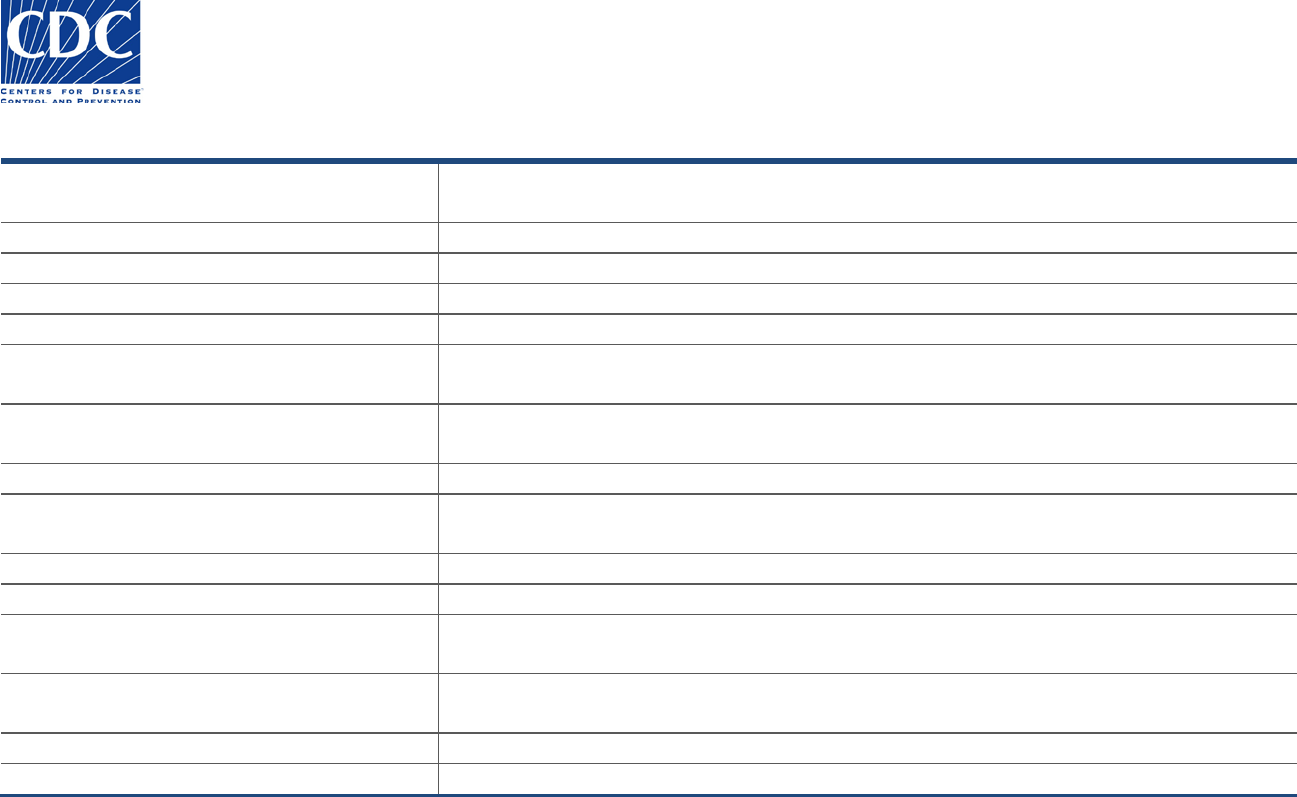
Intermediate Submitter
The Intermediate Submitter section appears below:
This section is used to enter the name, address, and contact information for the intermediate laboratory, which is usually the reference
laboratory that handled the sample (e.g., Quest, Lab Corp, ARUP, Mayo Clinic, and so on).
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 26 of 35
Field Name
Field Instructions
Name (last, first, MI, suffix, degree)
Name of person authorizing reference testing to be performed. This person is usually the
laboratory director or their designee.
Institution Name
Enter the institution name and specific department, if appropriate.
Street address 1
Enter the street address, including the specific floor/room number.
Street address 2
Enter the post office box or mailstop.
City, State, Zipcode, Country
Enter the city, state or province, zip or postal code, and country.
Phone (country code, area code, local
number, extension)
Enter local phone number for the laboratory, including country code and area code (numbers
only; no spaces or special characters).
Fax (country code, area code, local number)
Enter country code, area code, and local number in the appropriate fields (numbers only; no
spaces or special characters).
Institutional e-mail
Enter an email address for the institution or lab director.
Point of Contact (prefix, last, first, middle
initial, suffix, degree)
Enter the primary or alternative person in the laboratory who can answer questions
regarding the specimen submission.
Phone (country, area code, local number)
Enter the Point of Contact’s direct phone number
POC e-mail
Enter the Point of Contact’s direct email address
Sample ID
Enter the primary sample ID if assigned by the State PHL (SPHL). The number might be used
for studies.
Specimen ID
Enter the primary specimen ID if assigned by the SPHL. The number might be used for
studies.
Alternative Sample ID
Alternative sample ID if assigned by the SPHL.
Alternative Specimen ID
Alternative specimen ID if assigned by the SPHL.

50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 27 of 35
Specimen Identifiers (Auto Populated)
The Specimen Identifier section appears below:
This section is found at the top-most area on the second page of the form. The purpose of this section is to carry forward the specimen
identifiers that were entered on the front of the form. This is helpful in the event that the form is printed on two separate pieces of paper.
Field Name
Field Instructions
Original Specimen ID
Auto-populated from the Original Submitter section.
AND/OR Original Sample ID
Auto-populated from the Original Submitter section.
AND/OR SPHL Specimen ID
Auto-populated from the State PHL section
Caution: If you are not filling out the form using your computer, the Specimen
Identifier section will not auto populate. For printed forms, be sure to hand-write
the Original Specimen ID, Original Sample ID, and the SPHL Specimen ID in the
Specimen Identifier Section.

50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 28 of 35
Sample Information
The Sample Information section appears below:
This section is used to provide more information about
the sample and the business from which it was collected
which may be of public health importance.
Field Name
Field Instructions
Brand/Manufacturer
Enter the name of the manufacturer of the
product, e.g., ABC Company.
Specific Product
Name
Enter the specific name of the product, e.g.
ABC No-fuss Contact Lens Solution.
Lot Number
Enter the lot number of the product if
available, e.g., Lot No: 123-EZ-4436.
Expiration Date
Enter the expiration date for the product.
Quantity
Enter quantity of samples (aliquots)
submitted
Business Name
Enter the name of the business at which the
product was purchased.
Street address 1
Enter the street address, including the
specific floor/room number.
Street address 2
Enter the post office box or mailstop.
City
Enter the city.
Zip/Postal Code
Enter the zip code or postal code.
State
Select the state or province.
Country
Select the country.
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 29 of 35
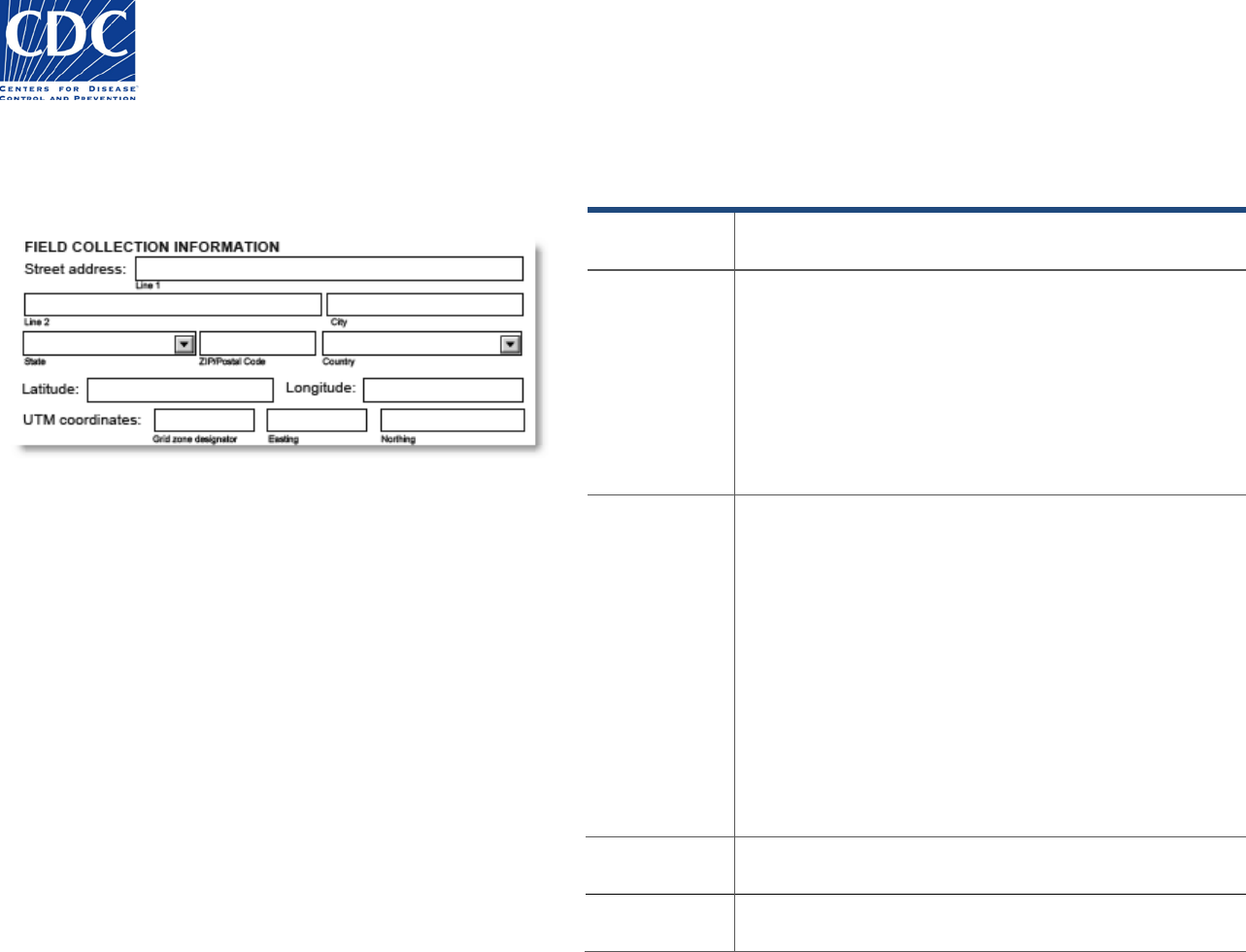
Sample Location Information
The Sample Location section appears below:
Food or environmental specimens may be collected in a field or
from a body of water. Information about the location at which
the specimen was collected is entered in these fields. Latitude
and longitude at which specimens are collected may be
recorded using Global Positioning System (GPS) Coordinates.
Field Name
Field Instructions
Sample
collected at
Enter the location name, e.g., Walden Pond.
Latitude /
Longitude
Global Positioning System (GPS) coordinates may be
used, if they have been documented for specimens that
have been collected at remote locations. GPS
coordinates are recorded in Common Geocoding
Format that is displayed in most GPS units. Examples of
positions are below:
Latitude may be recorded as N41 25.117
Longitude may be recorded as W83 58.292
UTM
Coordinates
(Grid Zone
Designator,
Easting,
Northing)
Universal Tranverse Mercator (UTM) coordinates may
be used as an alternative method for recording remote
locations where specimens have been collected.
Positions are defined by the following:
• Grid Zone Designator – This is a 2-digit number
that indicates the zone in which the specimen is
collected.
• Easting – This is a 6 to 8 digit number indicating
the east-west position.
Northing – This is a 6 to 8 digit number indicating the
north-south position.
Environmental
Temperature
Record the temperature of the environment from which
the sample was collected including the unit (e.g. ºC, ºF)
Sample
collected at
Enter the location name, e.g., Walden Pond.
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 30 of 35
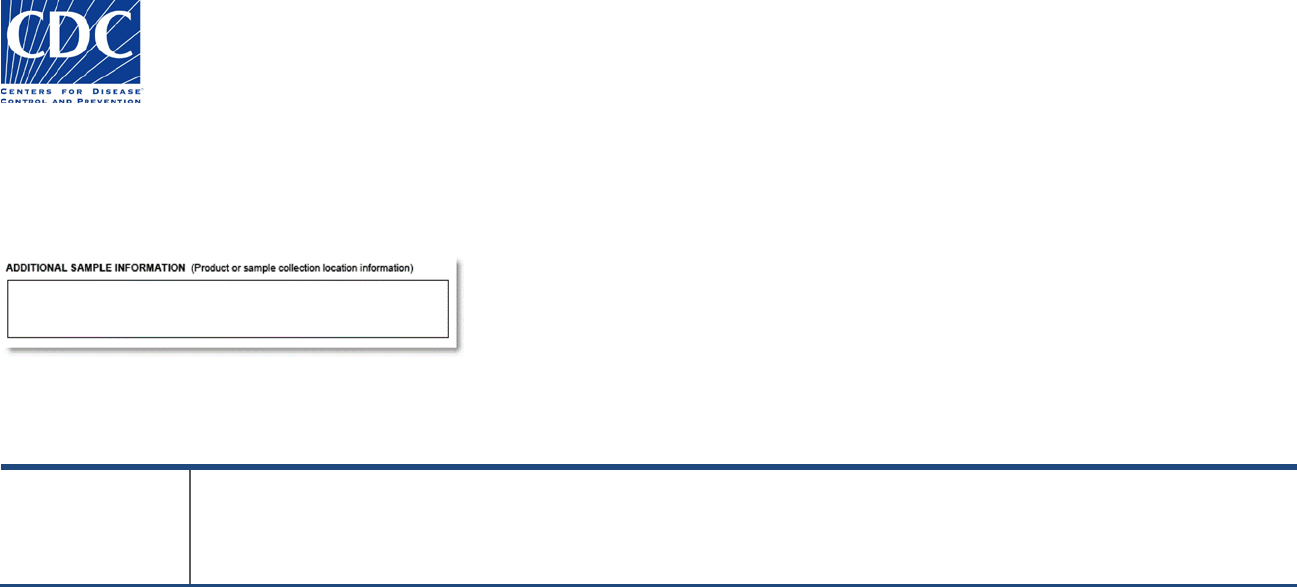
Additional Sample Information
The Additional Sample Information section appears below:
This section is used to provide information that has not been collected previously and which may be relevant for laboratories performing testing.
Field Name
Field Instructions
Additional Sample
Information
Provide any additional information regarding the sample that may be relevant for laboratories performing testing. The
character limit for this field is 250 characters. If the information does not fit in the field, then attach additional
documentation (e.g., worksheet) to the form.
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 31 of 35
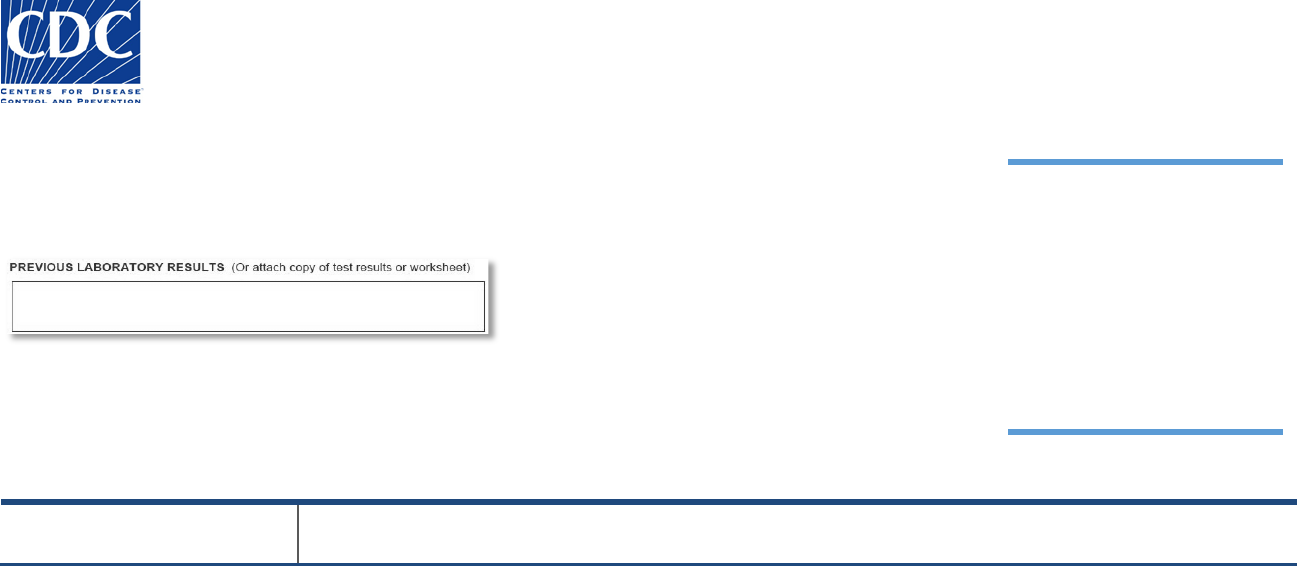
Previous Laboratory Results
The Previous Laboratory Results section appears below:
This section is used to document any previous laboratory results associated with this specimen. Additional
documentation such as test results may be attached to the form. Any additional information about the
submitted specimen can be captured in “Comments”
Field Name
Field Instructions
Previous Laboratory Results
Enter the sample’s previous laboratory results (250 character limit). If more space is needed, attach
additional documentation (e.g., test results, worksheet) to the form and/or continue under “Comments”.
Note: When attaching
additional documentation to
the form, please indicate that
you are attaching additional
information and note the
name of the attached
document in the Previous
Laboratory Results section.

50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 32 of 35
Comments
The Comments section appears below:
This section is used to document any additional information about the submitted specimen or when more space is required for other data fields.
CDC Use Only Barcodes
The image below depicts a CDC Use Only Barcode.
Field Name
Field Instructions
Comments
Enter additional information related to the specimen (250 character limit).
This field is also used to record data for fields where more space is required.

50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 33 of 35
Information that is entered into the form is recorded and saved in one of three barcode sections that only appear when the form prints
successfully. When a specimen is received at the CDC, the data from its corresponding Specimen Submission form is scanned via the barcodes
directly into the CDC Enterprise LIMS. This eliminates the need for data entry and reduces the amount of human error.
Field Name
Field Instructions
Barcode 1
Encodes information on page 1, left column
Barcode 2
Encodes information on page 1, right column
Barcode 3
Encodes information on page 2
Caution: The form must be filled out on your computer, printed and then sent to
the CDC with the specimen in order to take full advantage of the barcode
functionality. Information that is hand-written on the form will not be recorded
in the barcodes.
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 34 of 35
Expiring Template Forms
How to Obtain a Current Template Form
Overview
The Specimen Submission form contains a version number and
expiration in the footer, on the bottom right side of both sides of
the form. You will not be able to fill out the form or print the
form after the expiration date.
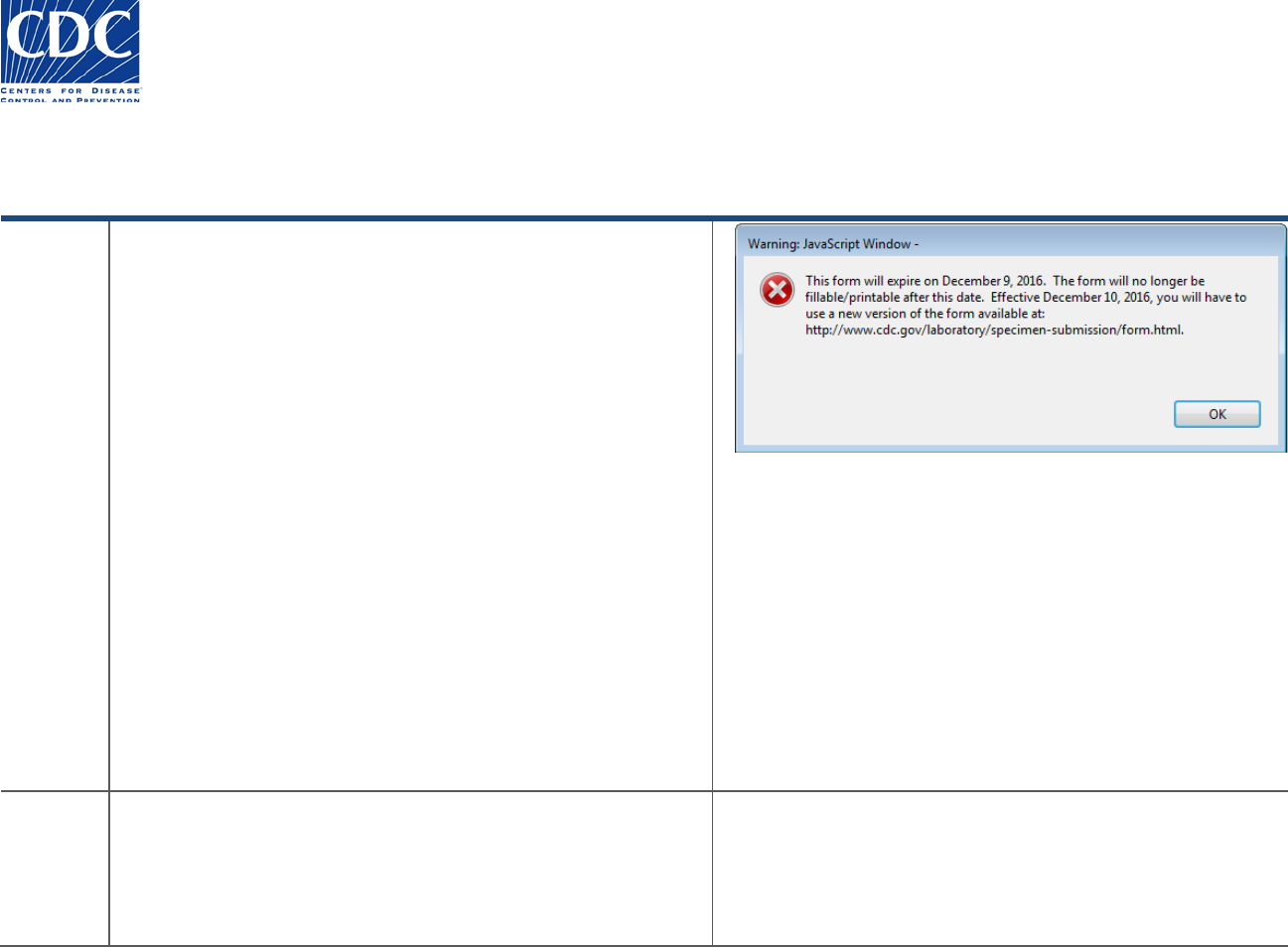
You will receive the following warning message each time you
open the form, beginning two weeks prior to the expiration date:
“This form will expire on ‘Month 99, 9999’. The form will no
longer be fillable/printable after this date. Effective ‘Month 99,
9999’ you will have to download a new version of the form at:
http://www.cdc.gov/laboratory/specimen-
submission/form.html”
If you open the form after the expiration date, you will receive
the following message:
“This form expired on ‘Month 99, 9999’. Effective ‘Month 99,
9999’, please use the new version of the form available at:
http://www.cdc.gov/laboratory/specimen-
submission/form.html”.
Action
Follow these steps to obtain a new form:
1. Discard all blank paper template forms, and blank template forms
stored on your computer that reflect the expiration date.
2. Download a new version of the template form at:
http://www.cdc.gov/laboratory/specimen-submission/form.html
Version 2.0, Expiration Date: 12/08/2017
50.34 version 2.0 | FEMB Specimen Submission Form Training Guide
Page 35 of 35
Overview
The Specimen Submission form contains a version number and
expiration in the footer, on the bottom right side of both sides of
the form. You will not be able to fill out the form or print the
form after the expiration date.
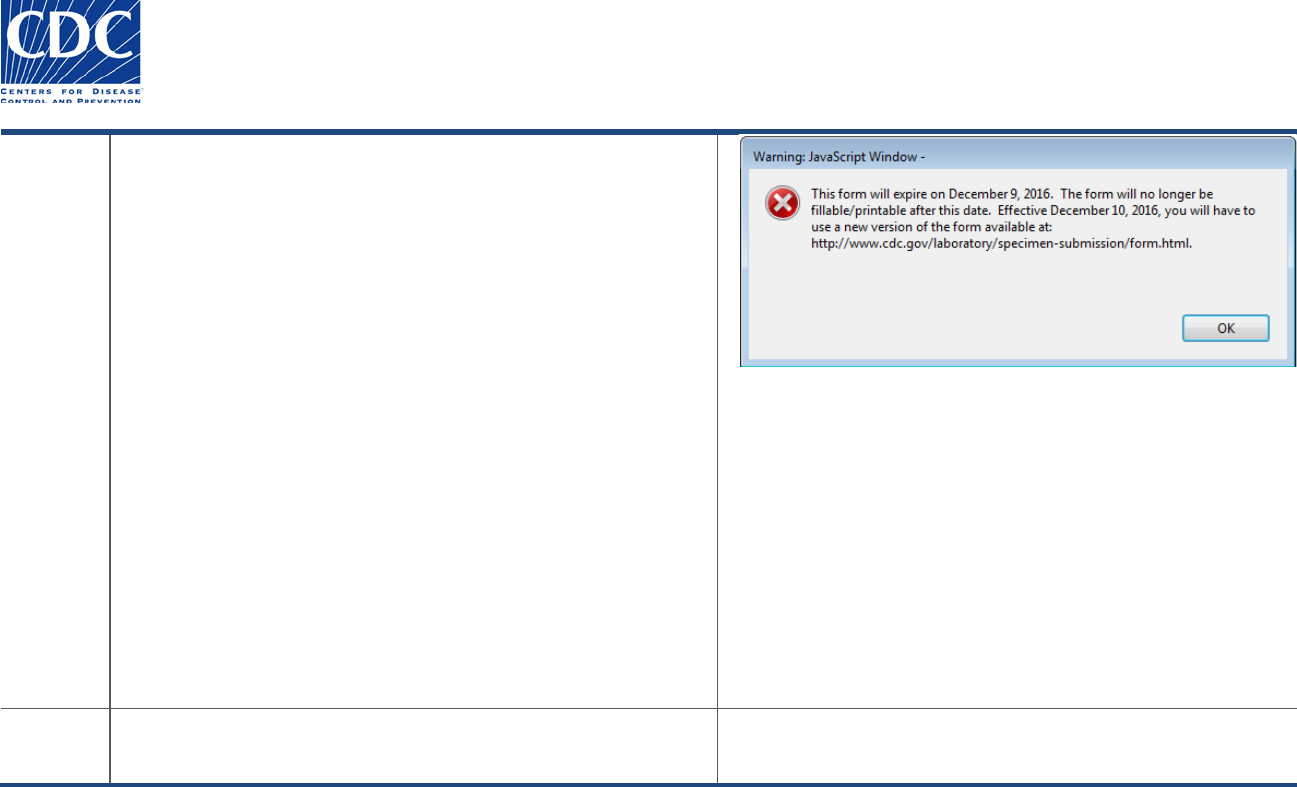
You will receive the following warning message each time you
open the form, beginning two weeks prior to the expiration date:
“This form will expire on ‘Month 99, 9999’. The form will no
longer be fillable/printable after this date. Effective ‘Month 99,
9999’ you will have to download a new version of the form at:
http://www.cdc.gov/laboratory/specimen-
submission/form.html”
If you open the form after the expiration date, you will receive
the following message:
“This form expired on ‘Month 99, 9999’. Effective ‘Month 99,
9999’, please use the new version of the form available at:
http://www.cdc.gov/laboratory/specimen-
submission/form.html”.
Result
The downloaded form should reflect the new expiration date in
the footer on the front and back of the form.
Version 2.0, Expiration Date: 12/08/2017