HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use
REVLIMID
®
safely and effectively. See full prescribing information for
REVLIMID.
REVLIMID (lenalidomide) capsules, for oral use
Initial U.S. Approval: 2005
WARNING: EMBRYO-FETAL TOXICITY, HEMATOLOGIC
TOXICITY, and VENOUS and ARTERIAL
THROMBOEMBOLISM
See full prescribing information for complete boxed warning.
EMBRYO-FETAL TOXICITY
Lenalidomide, a thalidomide analogue, caused limb
abnormalities in a developmental monkey study similar to birth
defects caused by thalidomide in humans. If lenalidomide is used
during pregnancy, it may cause birth defects or embryo-fetal
death.
Pregnancy must be excluded before start of treatment. Prevent
pregnancy during treatment by the use of two reliable methods
of contraception (5.1).
REVLIMID is available only through a restricted distribution
program, called the REVLIMID REMS
®
program (5.2, 17).
HEMATOLOGIC TOXICITY. REVLIMID can cause significant
neutropenia and thrombocytopenia (5.3).
VENOUS AND ARTERIAL THROMBOEMBOLISM
Significantly increased risk of deep vein thrombosis (DVT) and
pulmonary embolism (PE), as well as risk of myocardial
infarction and stroke in patients with multiple myeloma
receiving REVLIMID with dexamethasone. Anti-thrombotic
prophylaxis is recommended (5.4).
--------------------------RECENT MAJOR CHANGES----------------------------
Indications and Usage, Follicular Lymphoma (1.4) 5/19
Indications and Usage, Marginal Zone Lymphoma (1.5) 5/19
Dosage and Administration (2.4, 2.5) 5/19
---------------------------INDICATIONS AND USAGE----------------------------
REVLIMID is a thalidomide analogue indicated for the treatment of adult
patients with:
Multiple myeloma (MM), in combination with dexamethasone (1.1).
MM, as maintenance following autologous hematopoietic stem cell
transplantation (auto-HSCT) (1.1).
Transfusion-dependent anemia due to low- or intermediate-1-risk
myelodysplastic syndromes (MDS) associated with a deletion 5q
abnormality with or without additional cytogenetic abnormalities (1.2).
Mantle cell lymphoma (MCL) whose disease has relapsed or progressed
after two prior therapies, one of which included bortezomib (1.3).
Previously treated follicular lymphoma (FL), in combination with a
rituximab product (1.4).
Previously treated marginal zone lymphoma (MZL), in combination
with a rituximab product (1.5).
Limitations of Use:
REVLIMID is not indicated and is not recommended for the treatment
of patients with chronic lymphocytic leukemia (CLL) outside of
controlled clinical trials (1.4).
-----------------------DOSAGE AND ADMINISTRATION-----------------------
MM combination therapy: 25 mg once daily orally on Days 1-21 of
repeated 28-day cycles. (2.1).
MM maintenance therapy following auto-HSCT: 10 mg once daily
continuously on Days 1-28 of repeated 28-day cycles (2.1).
MDS: 10 mg once daily (2.2).
MCL: 25 mg once daily orally on Days 1-21 of repeated 28-day cycles
(2.3).
FL or MZL: 20 mg once daily orally on Days 1-21 of repeated 28-day
cycles for up to 12 cycles (2.4).
Renal impairment: Adjust starting dose based on the creatinine
clearance value (2.5).
For concomitant therapy doses, see Full Prescribing Information (2.1,
2.4, 14.1, 14.4).
----------------------DOSAGE FORMS AND STRENGTHS---------------------
Capsules: 2.5 mg, 5 mg, 10 mg, 15 mg, 20 mg, and 25 mg (3).
-------------------------------CONTRAINDICATIONS------------------------------
Pregnancy (Boxed Warning, 4.1, 5.1, 8.1).
Demonstrated severe hypersensitivity to lenalidomide (4.2, 5.9).
-----------------------WARNINGS AND PRECAUTIONS-----------------------
Increased mortality: serious and fatal cardiac adverse reactions occurred
in patients with CLL treated with REVLIMID (5.5).
Second Primary Malignancies (SPM): Higher incidences of SPM were
observed in controlled trials of patients with MM receiving REVLIMID
(5.6).
Increased Mortality: Observed in patients with MM when
pembrolizumab was added to dexamethasone and a thalidomide
analogue (5.7).
Hepatotoxicity: Hepatic failure including fatalities; monitor liver
function. Stop REVLIMID and evaluate if hepatotoxicity is suspected
(5.8).
Cutaneous Reactions, including fatalities: Hypersensitivity, angioedema,
Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction
with eosinophilia and systemic symptoms; discontinue REVLIMID if
reactions are suspected. Do not resume REVLIMID if these reactions are
verified (5.9).
Tumor lysis syndrome (TLS) including fatalities: Monitor patients at
risk of TLS (i.e., those with high tumor burden) and take appropriate
precautions (5.10).
Tumor flare reaction: Serious tumor flare reactions have occurred
during investigational use of REVLIMID for chronic lymphocytic
leukemia and lymphoma (5.11).
Impaired Stem Cell mobilization: A decrease in the number of CD34+
cells collected after treatment (> 4 cycles) with REVLIMID has been
reported. Consider early referral to transplant center (5.12).
Early mortality in MCL: Higher rate of early deaths have occurred in
patients with MCL (5.14).
-------------------------------ADVERSE REACTIONS------------------------------
MM: Most common adverse reactions (≥20%) include diarrhea, fatigue,
anemia, constipation, neutropenia, leukopenia, peripheral edema,
insomnia, muscle cramp/spasms, abdominal pain, back pain, nausea,
asthenia, pyrexia, upper respiratory tract infection, bronchitis,
nasopharyngitis, gastroenteritis, cough, rash, dyspnea, dizziness,
decreased appetite, thrombocytopenia, and tremor (6.1).
MDS: Most common adverse reactions (>15%) include
thrombocytopenia, neutropenia, diarrhea, pruritus, rash, fatigue,
constipation, nausea, nasopharyngitis, arthralgia, pyrexia, back pain,
peripheral edema, cough, dizziness, headache, muscle cramp, dyspnea,
pharyngitis, and epistaxis (6.1).
Non-Hodgkin’s Lymphoma (NHL: MCL, FL or MZL): Most common
adverse reactions (≥
15%) included neutropenia, thrombocytopenia,
anemia, leukopenia, diarrhea, constipation, nausea, fatigue, pyrexia,
cough, upper respiratory tract infection, and rash (6.1).
To report SUSPECTED ADVERSE REACTIONS contact Celgene
Corporation at 1-888-423-5436 or FDA at 1-800-FDA-1088 or
www.fda.gov/medwatch.
-------------------------------DRUG INTERACTIONS------------------------------
Digoxin: Monitor digoxin plasma levels periodically due to increased
C
max
and AUC with concomitant REVLIMID therapy (7.1).
Concomitant use of erythropoietin stimulating agents or estrogen
containing therapies with REVLIMID may increase the risk of
thrombosis (7.2).
--------------------------USE IN SPECIFIC POPULATIONS---------------------
Lactation: Advise not to breastfeed (8.2).
See 17 for PATIENT COUNSELING INFORMATION and Medication
Guide
Revised: 5/2019
Reference ID: 4439576
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: EMBRYO-FETAL TOXICITY, HEMATOLOGIC
TOXICITY, and VENOUS and ARTERIAL THROMBOEMBOLISM
1 INDICATIONS AND USAGE
1.1 Multiple Myeloma
1.2 Myelodysplastic Syndromes
1.3 Mantle Cell Lymphoma
1.4 Follicular Lymphoma
1.5 Marginal Zone Lymphoma
1.6 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Multiple Myeloma
2.2 Recommended Dosage for Myelodysplastic Syndromes
2.3 Recommended Dosage for Mantle Cell Lymphoma
2.4 Recommended Dosage for Follicular Lymphoma or Marginal Zone
Lymphoma
2.5 Recommended Dosage for Patients with Renal Impairment
2.6 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Pregnancy
4.2 Severe Hypersensitivity Reactions
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
5.2 REVLIMID REMS Program
5.3 Hematologic Toxicity
5.4 Venous and Arterial Thromboembolism
5.5 Increased Mortality in Patients with CLL
5.6 Second Primary Malignancies
5.7 Increased Mortality in Patients with MM When Pembrolizumab Is
Added to a Thalidomide Analogue and Dexamethasone
5.8 Hepatotoxicity
5.9 Severe Cutaneous Reactions Including Hypersensitivity Reactions
5.10 Tumor Lysis Syndrome
5.11 Tumor Flare Reaction
5.12 Impaired Stem Cell Mobilization
5.13 Thyroid Disorders
5.14 Early Mortality in Patients with MCL
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Digoxin
7.2 Concomitant Therapies That May Increase the Risk of Thrombosis
7.3 Warfarin
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Multiple Myeloma
14.2 Myelodysplastic Syndromes (MDS) with a Deletion 5q
Cytogenetic Abnormality
14.3 Mantle Cell Lymphoma
14.4 Follicular and Marginal Zone Lymphoma
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
16.3 Handling and Disposal
17 PATIENT COUNSELING INFORMATION
*Sections or subsections omitted from the full prescribing information are not
listed.
Reference ID: 4439576
2
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
FULL PRESCRIBING INFORMATION
WARNING: EMBRYO-FETAL TOXICITY, HEMATOLOGIC TOXICITY, and VENOUS and ARTERIAL THROMBOEMBOLISM
Embryo-Fetal Toxicity
Do not use REVLIMID during pregnancy. Lenalidomide, a thalidomide analogue, caused limb abnormalities in a developmental
monkey study. Thalidomide is a known human teratogen that causes severe life-threatening human birth defects. If lenalidomide is used
during pregnancy, it may cause birth defects or embryo-fetal death. In females of reproductive potential, obtain 2 negative pregnancy
tests before starting REVLIMID
®
treatment. Females of reproductive potential must use 2 forms of contraception or continuously
abstain from heterosexual sex during and for 4 weeks after REVLIMID treatment [see Warnings and Precautions (5.1), and Medication
Guide (17)]. To avoid embryo-fetal exposure to lenalidomide, REVLIMID is only available through a restricted distribution program,
the REVLIMID REMS
®
program (5.2).
Information about the REVLIMID REMS program is available at www.celgeneriskmanagement.com or by calling the manufacturer’s
toll-free number 1-888-423-5436.
Hematologic Toxicity (Neutropenia and Thrombocytopenia)
REVLIMID can cause significant neutropenia and thrombocytopenia. Eighty percent of patients with del 5q myelodysplastic syndromes
had to have a dose delay/reduction during the major study. Thirty-four percent of patients had to have a second dose delay/reduction.
Grade 3 or 4 hematologic toxicity was seen in 80% of patients enrolled in the study. Patients on therapy for del 5q myelodysplastic
syndromes should have their complete blood counts monitored weekly for the first 8 weeks of therapy and at least monthly thereafter.
Patients may require dose interruption and/or reduction. Patients may require use of blood product support and/or growth factors [see
Dosage and Administration (2.2)].
Venous and Arterial Thromboembolism
REVLIMID has demonstrated a significantly increased risk of deep vein thrombosis (DVT) and pulmonary embolism (PE), as well as
risk of myocardial infarction and stroke in patients with multiple myeloma who were treated with REVLIMID and dexamethasone
therapy. Monitor for and advise patients about signs and symptoms of thromboembolism. Advise patients to seek immediate medical
care if they develop symptoms such as shortness of breath, chest pain, or arm or leg swelling. Thromboprophylaxis is recommended and
the choice of regimen should be based on an assessment of the patient’s underlying risks [see Warnings and Precautions (5.4)].
1 INDICATIONS AND USAGE
1.1 Multiple Myeloma
REVLIMID in combination with dexamethasone is indicated for the treatment of adult patients with multiple myeloma (MM).
REVLIMID is indicated as maintenance therapy in adult patients with MM following autologous hematopoietic stem cell transplantation (auto-HSCT).
1.2 Myelodysplastic Syndromes
REVLIMID is indicated for the treatment of adult patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes (MDS)
associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities.
1.3 Mantle Cell Lymphoma
REVLIMID is indicated for the treatment of adult patients with mantle cell lymphoma (MCL) whose disease has relapsed or progressed after two prior therapies, one of
which included bortezomib.
1.4 Follicular Lymphoma
REVLIMID in combination with a rituximab product, is indicated for the treatment of adult patients with previously treated follicular lymphoma (FL).
1.5 Marginal Zone Lymphoma
REVLIMID in combination with a rituximab product, is indicated for the treatment of adult patients with previously treated marginal zone lymphoma (MZL).
1.6 Limitations of Use
REVLIMID is not indicated and is not recommended for the treatment of patients with CLL outside of controlled clinical trials [see Warnings and Precautions (5.5)].
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Multiple Myeloma
REVLIMID Combination Therapy
The recommended starting dose of REVLIMID is 25 mg orally once daily on Days 1-21 of repeated 28-day cycles in combination with dexamethasone. Refer to
Section 14.1 for specific dexamethasone dosing. For patients greater than 75 years old, the starting dose of dexamethasone may be reduced [see Clinical Studies
(14.1)]. Treatment should be continued until disease progression or unacceptable toxicity.
In patients who are not eligible for auto-HSCT, treatment should continue until disease progression or unacceptable toxicity. For patients who are auto-HSCT-eligible,
hematopoietic stem cell mobilization should occur within 4 cycles of a REVLIMID-containing therapy [see Warnings and Precautions (5.12)].
Dose Adjustments for Hematologic Toxicities During MM Treatment
Dose modification guidelines, as summarized in Table 1 below, are recommended to manage Grade 3 or 4 neutropenia or thrombocytopenia or other Grade 3 or 4
toxicity judged to be related to REVLIMID.
Table 1: Dose Adjustments for Hematologic Toxicities for MM
Reference ID: 4439576
3
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Platelet counts
Thrombocytopenia in MM
When Platelets Recommended Course
Days 1-21 of repeated 28-day cycle
Fall below 30,000/mcL Interrupt REVLIMID treatment, follow CBC weekly
Return to at least 30,000/mcL Resume REVLIMID at next lower dose. Do not dose
b
elow 2.5 mg daily
For each subsequent drop below 30,000/mcL Interrupt REVLIMID treatmen
t
Return to at least 30,000/mcL Resume REVLIMID at next lower dose. Do not dose
b
elow 2.5 mg daily
Absolute Neutrophil counts (ANC)
Neutropenia in MM
When Neutrophils Recommended Course
Days 1-21 of repeated 28-day cycle
Fall below 1000/mcL Interrupt REVLIMID treatment, follow CBC
weekly
Return to at least 1,000/mcL and neutropenia is the only toxicity Resume REVLIMID at 25 mg daily or initial
starting dose
Return to at least 1,000/mcL and if other toxicity Resume REVLIMID at next lower dose. Do not
dose below 2.5 mg daily
For each subsequent drop below 1,000/mcL Interrupt REVLIMID treatmen
t
Return to at least 1,000/mcL Resume REVLIMID at next lower dose. Do not
dose below 2.5 mg daily
REVLIMID Maintenance Therapy Following Auto-HSCT
Following auto-HSCT, initiate REVLIMID maintenance therapy after adequate hematologic recovery (ANC at least 1000/mcL and/or platelet counts at least
75,000/mcL). The recommended starting dose of REVLIMID is 10 mg once daily continuously (Days 1-28 of repeated 28-day cycles) until disease progression or
unacceptable toxicity. After 3 cycles of maintenance therapy, the dose can be increased to 15 mg once daily if tolerated.
Dose Adjustments for Hematologic Toxicities During MM Treatment
Dose modification guidelines, as summarized in Table 2 below, are recommended to manage Grade 3 or 4 neutropenia or thrombocytopenia or other Grade 3 or 4
toxicity judged to be related to REVLIMID.
Table 2: Dose Adjustments for Hematologic Toxicities for MM
Platelet counts
Thrombocytopenia in MM
When Platelets Recommended Course
Fall below 30,000/mcL
Return to at least 30,000/mcL
If at the 5 mg daily dose,
For a subsequent drop below 30,000/mcL
Interrupt REVLIMID treatment, follow CBC weekly
Resume REVLIMID at next lower dose, continuously
for Days 1-28 of repeated 28-day cycle
Interrupt REVLIMID treatment. Do not dose below 5
mg daily for Day 1 to 21 of 28 day cycle
Return to at least 30,000/mcL
Absolute Neutrophil counts (ANC)
Resume REVLIMID at 5 mg daily for Days 1 to 21of
28-day cycle. Do not dose below 5 mg daily for Day 1
to 21 of 28 day cycle
Neutropenia in MM
When Neutrophils
Fall below 500/mcL
Return to at least 500/mcL
If at 5 mg daily dose,
For a subsequent drop below 500/mcL
Recommended Course
Interrupt REVLIMID treatment, follow CBC weekly
Resume REVLIMID at next lower dose,
continuously for Days 1-28 of repeated 28-day cycle
Interrupt REVLIMID treatment. Do not dose below 5
mg daily for Days 1 to 21 of 28-day cycle
Return to at least 500/mcL Resume REVLIMID at 5 mg daily for Days 1 to 21 of
28-day cycle. Do not dose below 5 mg daily for Days
1 to 21 of 28-day cycle
Other Toxicities in MM
For other Grade 3/4 toxicities judged to be related to REVLIMID, hold treatment and restart at the physician's discretion at next lower dose level when toxicity has
resolved to Grade 2 or lower.
Starting Dose Adjustment for Renal Impairment in MM
[see Dosage and Administration (2.5)].
Recommended Dosage for Myelodysplastic Syndromes
Reference ID: 4439576
4
2.2
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

The recommended starting dose of REVLIMID is 10 mg daily. Treatment is continued or modified based upon clinical and laboratory findings. Continue treatment
until disease progression or unacceptable toxicity.
Dose Adjustments for Hematologic Toxicities During MDS Treatment
Patients who are dosed initially at 10 mg and who experience thrombocytopenia should have their dosage adjusted as follows:
Platelet counts
If thrombocytopenia develops WITHIN 4 weeks of starting treatment at 10 mg daily in MDS
If baseline is at least 100,000/mcL
When Platelets Recommended Course
Fall below 50,000/mcL Interrupt REVLIMID treatmen
t
Return to at least 50,000/mcL Resume REVLIMID at 5 mg daily
If baseline is below 100,000/mcL
When Platelets Recommended Course
Fall to 50% of the baseline value Interrupt REVLIMID treatmen
t
If baseline is at least 60,000/mcL and Resume REVLIMID at 5 mg daily
returns to at least 50,000/mcL
If baseline is below 60,000/mcL and Resume REVLIMID at 5 mg daily
returns to at least 30,000/mcL
If thrombocytopenia develops AFTER 4 weeks of starting treatment at 10 mg daily in MDS
When Platelets Recommended Course
Fall below 30,000/mcL or below 50,000/mcL Interrupt REVLIMID treatment
with platelet transfusions
Return to at least 30,000/mcL Resume REVLIMID at 5 mg daily
(without hemostatic failure)
Patients who experience thrombocytopenia at 5 mg daily should have their dosage adjusted as follows:
If thrombocytopenia develops during treatment at 5 mg daily in MDS
When Platelets Recommended Course
Fall below 30,000/mcL or below 50,000/mcL Interrupt REVLIMID treatment
with platelet transfusions
Return to at least 30,000/mcL Resume REVLIMID at 2.5 mg daily
(without hemostatic failure)
Patients who are dosed initially at 10 mg and experience neutropenia should have their dosage adjusted as follows:
Absolute Neutrophil counts (ANC)
If neutropenia develops WITHIN 4 weeks of starting treatment at 10 mg daily in MDS
If baseline ANC is at least 1,000/mcL
When Neutrophils Recommended Course
Fall below 750/mcL Interrupt REVLIMID treatmen
t
Return to at least 1,000/mcL Resume REVLIMID at 5 mg daily
If baseline ANC is below 1,000/mcL
When Neutrophils Recommended Course
Fall below 500/mcL Interrupt REVLIMID treatmen
t
Return to at least 500/mcL Resume REVLIMID at 5 mg daily
If neutropenia develops AFTER 4 weeks of starting treatment at 10 mg daily in MDS
When Neutrophils Recommended Course
Fall below 500/mcL for at least 7 days or below 500/mcL Interrupt REVLIMID treatment
associated with fever (at least 38.5°C)
Return to at least 500/mcL Resume REVLIMID at 5 mg daily
Patients who experience neutropenia at 5 mg daily should have their dosage adjusted as follows:
If neutropenia develops during treatment at 5 mg daily in MDS
When Neutrophils Recommended Course
Fall below 500/mcL for at least 7 days or below 500/mcL Interrupt REVLIMID treatment
associated with fever (at least 38.5°C)
Return to at least 500/mcL Resume REVLIMID at 2.5 mg daily
Reference ID: 4439576
5
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

2.3
2.4
Other Grade 3 / 4 Toxicities in MDS
For other Grade 3/4 toxicities judged to be related to REVLIMID, hold treatment and restart at the physician's discretion at next lower dose level when toxicity has
resolved to Grade 2 or lower.
Starting Dose Adjustment for Renal Impairment in MDS
[see Dosage and Administration (2.5)].
Recommended Dosage for Mantle Cell Lymphoma
The recommended starting dose of REVLIMID is 25 mg/day orally on Days 1-21 of repeated 28-day cycles for relapsed or refractory mantle cell lymphoma.
Treatment should be continued until disease progression or unacceptable toxicity.
Treatment is continued, modified or discontinued based upon clinical and laboratory findings.
Dose Adjustments for Hematologic Toxicities During MCL Treatment
Dose modification guidelines as summarized below are recommended to manage Grade 3 or 4 neutropenia or thrombocytopenia or other Grade 3 or 4 toxicities
considered to be related to REVLIMID.
Platelet counts
Thrombocytopenia during treatment in MCL
When Platelets
Fall below 50,000/mcL
Recommended Course
Interrupt REVLIMID treatment and follow CBC weekly
Return to at least 50,000/mcL
Resume REVLIMID at 5 mg less than the previous dose. Do
not dose
b
elow 5 mg daily
Absolute Neutrophil counts (ANC)
Neutropenia during treatment in MCL
When Neutrophils
Fall below 1000/mcL for at least 7 days
OR
Falls below 1,000/mcL with an associated temperature at least
38.5°C
OR
Falls
b
elow 500/mcL
Recommended Course
Interrupt REVLIMID treatment and follow CBC weekly
Return to at least 1,000/mcL Resume REVLIMID at 5 mg less than the previous dose. Do
not dose below 5 mg daily
Other Grade 3 / 4 Toxicities in MCL
For other Grade 3/4 toxicities judged to be related to REVLIMID, hold treatment and restart at the physician’s discretion at next lower dose level when toxicity has
resolved to Grade 2 or lower.
Starting Dose Adjustment for Renal Impairment in MCL
[see Dosage and Administration (2.5)].
Recommended Dosage for Follicular Lymphoma or Marginal Zone Lymphoma
The recommended starting dose of REVLIMID is 20 mg orally once daily on Days 1-21 of repeated 28-day cycles for up to 12 cycles of treatment in combination with
a rituximab-product. Refer to Section 14.4 for specific rituximab dosing from the AUGMENT trial. For dose adjustments due to toxicity with rituximab, refer to the
product prescribing information.
Dose Adjustments for Hematologic Toxicities during FL or MZL Treatment
Dose modification guidelines, as summarized below, are recommended to manage Grade 3 or 4 neutropenia or thrombocytopenia or other Grade 3 or 4 toxicity judged
to be related to REVLIMID.
Platelet counts
Thrombocytopenia during treatment in FL or MZL
When Platelets
Fall below 50,000/mcL
Return to at least 50,000/mcL
Recommended Course
Interrupt REVLIMID treatment and follow CBC weekly.
If patient starting dose was 20 mg daily, resume REVLIMID
at 5 mg less than the previous dose. Do not dose below 5 mg
daily.
If patient starting dose was 10 mg daily, resume at 5 mg less
than previous dose. Do not dose below 2.5 mg daily.
Absolute Neutrophil counts (ANC)
Neutropenia during treatment in FL or MZL
6
Reference ID: 4439576
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

2.5
When Neutrophils Recommended Course
Fall below 1,000/mcL for at least 7 days Interrupt REVLIMID treatment and follow CBC weekly.
OR
Falls below 1,000/mcL with an associated temperature at least
38.5°C
OR
Falls below 500 /mcL
Return to at least 1,000/mcL If patient starting dose was 20 mg daily, resume REVLIMID
at 5 mg less than the previous dose. Do not dose below 5 mg
daily.
If patient starting dose was 10 mg daily, resume at 5 mg less
than previous dose. Do not dose below 2.5 mg daily.
Other Grade 3 / 4 Toxicities in FL or MZL
For other Grade 3/4 toxicities judged to be related to REVLIMID, hold treatment and restart at the physician's discretion at next lower dose level when toxicity has
resolved to Grade 2 or below.
Starting Dose Adjustment for Renal Impairment in FL or MZL
[see Dosage and Administration (2.5)].
Recommended Dosage for Patients with Renal Impairment
The recommendations for dosing patients with renal impairment are shown in the following table [see Clinical Pharmacology (12.3)].
Table 3: Dose Adjustments for Patients with Renal Impairment
Renal Function
(Cockcroft-Gault)
Dose in REVLIMID Combination
Therapy for MM and MCL
Dose in REVLIMID Combination
Therapy for FL and MZL
Dose in REVLIMID Maintenance
Therapy Following Auto-HSCT for
MM and for MDS
CLcr 30 to 60 mL/min 10 mg once daily 10 mg once daily 5 mg once daily
CLcr below 30 mL/min (not requiring
dialysis)
15 mg every other day 5 mg once daily 2.5 mg once daily
CLcr below 30 mL/min (requiring
dialysis)
5 mg once daily. On dialysis days,
administer the dose following
dialysis.
5 mg once daily. On dialysis days,
administer the dose following
dialysis.
2.5 mg once daily. On dialysis days,
administer the dose following
dialysis.
REVLIMID Combination Therapy for MM: For CLcr of 30 to 60 mL/min, consider escalating the dose to 15 mg after 2 cycles if the patient tolerates the 10 mg dose of
lenalidomide without dose-limiting toxicity.
REVLIMID Maintenance Therapy Following Auto-HSCT for MM and for MCL and MDS: Base subsequent REVLIMID dose increase or decrease on individual
patient treatment tolerance [see Dosage and Administration (2.1- 2.3)].
REVLIMID Combination Therapy for FL or for MZL: For patients with CLcr of 30 to 60 mL/min, after 2 cycles, the REVLIMID dose may be increased to 15 mg
orally if the patient has tolerated therapy.
2.6 Administration
Advise patients to take REVLIMID orally at about the same time each day, either with or without food. Advise patients to swallow REVLIMID capsules whole with
water and not to open, break, or chew them.
3 DOSAGE FORMS AND STRENGTHS
Capsules:
2.5 mg, white and blue-green opaque hard capsules imprinted “REV” on one half and “2.5 mg” on the other half in black ink
5 mg, white opaque capsules imprinted “REV” on one half and “5 mg” on the other half in black ink
10 mg, blue/green and pale yellow opaque capsules imprinted “REV” on one half and “10 mg” on the other half in black ink
15 mg, powder blue and white opaque capsules imprinted “REV” on one half and “15 mg” on the other half in black ink
20 mg, powder blue and blue-green opaque hard capsules imprinted “REV” on one half and “20 mg” on the other half in black ink
25 mg, white opaque capsules imprinted “REV” on one half and “25 mg” on the other half in black ink
4 CONTRAINDICATIONS
4.1 Pregnancy
REVLIMID can cause fetal harm when administered to a pregnant female. Limb abnormalities were seen in the offspring of monkeys that were dosed with
lenalidomide during organogenesis. This effect was seen at all doses tested. Due to the results of this developmental monkey study, and lenalidomide’s structural
similarities to thalidomide, a known human teratogen, lenalidomide is contraindicated in females who are pregnant [see Boxed Warning]. If this drug is used during
pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential risk to a fetus [see Warnings and Precautions (5.1,
5.2), Use in Special Populations (8.1, 8.3)].
Reference ID: 4439576
7
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
4.2 Severe Hypersensitivity Reactions
REVLIMID is contraindicated in patients who have demonstrated severe hypersensitivity (e.g., angioedema, Stevens-Johnson syndrome, toxic epidermal necrolysis) to
lenalidomide [see Warnings and Precautions (5.8)].
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
REVLIMID is a thalidomide analogue and is contraindicated for use during pregnancy. Thalidomide is a known human teratogen that causes life-threatening human
birth defects or embryo-fetal death [see Use in Specific Populations (8.1)]. An embryo-fetal development study in monkeys indicates that lenalidomide produced
malformations in the offspring of female monkeys who received the drug during pregnancy, similar to birth defects observed in humans following exposure to
thalidomide during pregnancy.
REVLIMID is only available through the REVLIMID REMS program [see Warnings and Precautions (5.2)].
Females of Reproductive Potential
Females of reproductive potential must avoid pregnancy for at least 4 weeks before beginning REVLIMID therapy, during therapy, during dose interruptions and for at
least 4 weeks after completing therapy.
Females must commit either to abstain continuously from heterosexual sexual intercourse or to use two methods of reliable birth control, beginning 4 weeks prior to
initiating treatment with REVLIMID, during therapy, during dose interruptions and continuing for 4 weeks following discontinuation of REVLIMID therapy.
Two negative pregnancy tests must be obtained prior to initiating therapy. The first test should be performed within 10-14 days and the second test within 24 hours
prior to prescribing REVLIMID therapy and then weekly during the first month, then monthly thereafter in females with regular menstrual cycles or every 2 weeks in
females with irregular menstrual cycles [see Use in Specific Populations (8.3)].
Males
Lenalidomide is present in the semen of patients receiving the drug. Therefore, males must always use a latex or synthetic condom during any sexual contact with
females of reproductive potential while taking REVLIMID and for up to 4 weeks after discontinuing REVLIMID, even if they have undergone a successful vasectomy.
Male patients taking REVLIMID must not donate sperm [see Use in Specific Populations (8.3)].
Blood Donation
Patients must not donate blood during treatment with REVLIMID and for 4 weeks following discontinuation of the drug because the blood might be given to a pregnant
female patient whose fetus must not be exposed to REVLIMID.
5.2 REVLIMID REMS Program
Because of the embryo-fetal risk [see Warnings and Precautions (5.1)], REVLIMID is available only through a restricted program under a Risk Evaluation and
Mitigation Strategy (REMS), the REVLIMID REMS program.
Required components of the REVLIMID REMS program include the following:
Prescribers must be certified with the REVLIMID REMS program by enrolling and complying with the REMS requirements.
Patients must sign a Patient-Physician agreement form and comply with the REMS requirements. In particular, female patients of reproductive potential who are not
pregnant must comply with the pregnancy testing and contraception requirements [see Use in Specific Populations (8.3)] and males must comply with contraception
requirements [see Use in Specific Populations (8.3)].
Pharmacies must be certified with the REVLIMID REMS program, must only dispense to patients who are authorized to receive REVLIMID and comply with
REMS requirements.
Further information about the REVLIMID REMS program is available at www.celgeneriskmanagement.com or by telephone at 1-888-423-5436.
Reference ID: 4439576
8
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
5.3 Hematologic Toxicity
REVLIMID can cause significant neutropenia and thrombocytopenia. Monitor patients with neutropenia for signs of infection. Advise patients to observe for bleeding
or bruising, especially with use of concomitant medication that may increase risk of bleeding. Patients taking REVLIMID should have their complete blood counts
assessed periodically as described below [see Dosage and Administration (2.1, 2.2, 2.3)].
Monitor complete blood counts (CBC) in patients taking REVLIMID in combination with dexamethasone or as REVLIMID maintenance therapy for MM every 7 days
(weekly) for the first 2 cycles, on Days 1 and 15 of Cycle 3, and every 28 days (4 weeks) thereafter. A dose interruption and/or dose reduction may be required [see
Dosage and Administration (2.1)]. In the MM maintenance therapy trials, Grade 3 or 4 neutropenia was reported in up to 59% of REVLIMID-treated patients and
Grade 3 or 4 thrombocytopenia in up to 38% of REVLIMID-treated patients [see Adverse Reactions (6.1)].
Monitor complete blood counts (CBC) in patients taking REVLIMID for MDS weekly for the first 8 weeks and at least monthly thereafter. Grade 3 or 4 hematologic
toxicity was seen in 80% of patients enrolled in the MDS study. In the 48% of patients who developed Grade 3 or 4 neutropenia, the median time to onset was 42 days
(range, 14-411 days), and the median time to documented recovery was 17 days (range, 2-170 days). In the 54% of patients who developed Grade 3 or 4
thrombocytopenia, the median time to onset was 28 days (range, 8-290 days), and the median time to documented recovery was 22 days (range, 5-224 days) [see Boxed
Warning and Dosage and Administration (2.2)].
Monitor complete blood counts (CBC) in patients taking REVLIMID for MCL weekly for the first cycle (28 days), every 2 weeks during cycles 2-4, and then monthly
thereafter. Patients may require dose interruption and/or dose reduction. In the MCL trial, Grade 3 or 4 neutropenia was reported in 43% of the patients. Grade 3 or 4
thrombocytopenia was reported in 28% of the patients.
Monitor complete blood counts (CBC) in patients taking REVLIMID for FL or MZL weekly for the first 3 weeks of Cycle 1 (28 days), every 2 weeks during Cycles 2-
4, and then monthly thereafter. Patients may require dose interruption and/or dose reduction. In the AUGMENT and MAGNIFY trials, Grade 3 or 4 neutropenia was
reported in 50% and 33%, respectively, of patients in the REVLIMID/rituximab arm. Grade 3 or 4 thrombocytopenia was reported in 2% and 8%, respectively, of
patients in the REVLIMID/rituximab arm [see Adverse Reactions (6.1)].
5.4 Venous and Arterial Thromboembolism
Venous thromboembolic events (VTE [DVT and PE]) and arterial thromboembolic events (ATE, myocardial infarction and stroke) are increased in patients treated with
REVLIMID.
A significantly increased risk of DVT (7.4%) and of PE (3.7%) occurred in patients with MM after at least one prior therapy who were treated with REVLIMID and
dexamethasone therapy compared to patients treated in the placebo and dexamethasone group (3.1% and 0.9%) in clinical trials with varying use of anticoagulant
therapies. In the newly diagnosed multiple myeloma (NDMM) study in which nearly all patients received antithrombotic prophylaxis, DVT was reported as a serious
adverse reaction (3.6%, 2.0%, and 1.7%) in the Rd Continuous, Rd18, and MPT Arms, respectively. The frequency of serious adverse reactions of PE was similar
between the Rd Continuous, Rd18, and MPT Arms (3.8%, 2.8%, and 3.7%, respectively) [see Boxed Warning and Adverse Reactions (6.1)].
Myocardial infarction (1.7%) and stroke (CVA) (2.3%) are increased in patients with MM after at least one prior therapy who were treated with REVLIMID and
dexamethasone therapy compared to patients treated with placebo and dexamethasone (0.6%, and 0.9%) in clinical trials. In the NDMM study, myocardial infarction
(including acute) was reported as a serious adverse reaction (2.3%, 0.6%, and 1.1%) in the Rd Continuous, Rd18, and MPT Arms, respectively. The frequency of
serious adverse reactions of CVA was similar between the Rd Continuous, Rd18, and MPT Arms (0.8%, 0.6 %, and 0.6%, respectively) [see Adverse Reactions (6.1)].
Patients with known risk factors, including prior thrombosis, may be at greater risk and actions should be taken to try to minimize all modifiable factors (e.g.
hyperlipidemia, hypertension, smoking).
In controlled clinical trials that did not use concomitant thromboprophylaxis, 21.5% overall thrombotic events (Standardized MedDRA Query Embolic and Thrombotic
events) occurred in patients with refractory and relapsed MM who were treated with REVLIMID and dexamethasone compared to 8.3% thrombosis in patients treated
with placebo and dexamethasone. The median time to first thrombosis event was 2.8 months. In the NDMM study in which nearly all pati
ents received antithrombotic
prophylaxis, the overall frequency of thrombotic events was 17.4% in patients in the combined Rd Continuous and Rd18 Arms, and was 11.6% in the MPT Arm. The
median time to first thrombosis event was 4.3 months in the combined Rd Continuous and Rd18 Arms.
In the AUGMENT trial, the incidence of VTE (including DVT and PE) in FL or MZL patients was 3.4% in the REVLIMID/rituximab arm [see Adverse Reactions
(6.1)]. In the AUGMENT trial, the incidence of ATE (including MI) in FL or MZL patients was 0.6% in the REVLIMID/rituximab arm [see Adverse Reactions (6.1)].
Thromboprophylaxis is recommended. The regimen of thromboprophylaxis should be based on an assessment of the patient’s underlying risks. Instruct patients to
report immediately any signs and symptoms suggestive of thrombotic events. ESAs and estrogens may further increase the risk of thrombosis and their use should be
based on a benefit-risk decision in patients receiving REVLIMID [see Drug Interactions (7.2)].
5.5 Increased Mortality in Patients with CLL
In a prospective randomized (1:1) clinical trial in the first line treatment of patients with chronic lymphocytic leukemia, single agent REVLIMID therapy increased the
risk of death as compared to single agent chlorambucil. In an interim analysis, there were 34 deaths among 210 patients on the REVLIMID treatment arm compared to
18 deaths among 211 patients in the chlorambucil treatment arm, and hazard ratio for overall survival was 1.92 [95% CI: 1.08 – 3.41], consistent with a 92% increase in
the risk of death. The trial was halted for safety in July 2013.
Serious adverse cardiovascular reactions, including atrial fibrillation, myocardial infarction, and cardiac failure occurred more frequently in the REVLIMID treatment
arm. REVLIMID is not indicated and not recommended for use in CLL outside of controlled clinical trials.
5.6 Second Primary Malignancies
In clinical trials in patients with MM receiving REVLIMID, an increase of hematologic plus solid tumor second primary malignancies (SPM) notably AML and MDS
have been observed. An increase in hematologic SPM including AML and MDS occurred in 5.3% of patients with NDMM receiving REVLIMID in combination with
oral melphalan compared with 1.3% of patients receiving melphalan without REVLIMID. The frequency of AML and MDS cases in patients with NDMM treated with
REVLIMID in combination with dexamethasone without melphalan was 0.4%.
In patients receiving REVLIMID maintenance therapy following high dose intravenous melphalan and auto-HSCT, hematologic SPM occurred in 7.5% of patients
compared to 3.3% in patients receiving placebo. The incidence of hematologic plus solid tumor (excluding squamous cell carcinoma and basal cell carcinoma) SPM
was 14.9%, compared to 8.8% in patients receiving placebo with a median follow-up of 91.5 months. Non-melanoma skin cancer SPM, including squamous cell
carcinoma and basal cell carcinoma, occurred in 3.9% of patients receiving REVLIMID maintenance, compared to 2.6% in the placebo arm.
Reference ID: 4439576
9
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
In patients with relapsed or refractory MM treated with REVLIMID/dexamethasone, the incidence of hematologic plus solid tumor (excluding squamous cell
carcinoma and basal cell carcinoma) SPM was 2.3% versus 0.6% in the dexamethasone alone arm. Non-melanoma skin cancer SPM, including squamous cell
carcinoma and basal cell carcinoma, occurred in 3.1% of patients receiving REVLIMID/dexamethasone, compared to 0.6% in the dexamethasone alone arm.
Patients who received REVLIMID-containing therapy until disease progression did not show a higher incidence of invasive SPM than patients treated in the fixed
duration REVLIMID-containing arms. Monitor patients for the development of second primary malignancies. Take into account both the potential benefit of
REVLIMID and the risk of second primary malignancies when considering treatment with REVLIMID.
In the AUGMENT trial with FL or MZL patients receiving REVLIMID/rituximab therapy, hematologic plus solid tumor SPMs, notably AML, have been observed. In
the AUGMENT trial, hematologic SPM of AML occurred in 0.6% of patients with FL or MZL receiving REVLIMID/rituximab therapy. The incidence of hematologic
plus solid tumor SPMs (excluding nonmelanoma skin cancers) was 1.7% in the REVLIMID/rituximab arm with a median follow-up of 29.8 months (range 0.5 to 51.3
months) [see Adverse Reactions (6.1)]. Monitor patients for the development of second primary malignancies. Take into account both the potential benefit of
REVLIMID and the risk of second primary malignancies when considering treatment with REVLIMID.
5.7 Increased Mortality in Patients with MM When Pembrolizumab Is Added to a Thalidomide Analogue and Dexamethasone
In two randomized clinical trials in patients with MM, the addition of pembrolizumab to a thalidomide analogue plus dexamethasone, a use for which no PD-1 or
PD-L1 blocking antibody is indicated, resulted in increased mortality. Treatment of patients with MM with a PD-1 or PD-L1 blocking antibody in combination with a
thalidomide analogue plus dexamethasone is not recommended outside of controlled clinical trials.
5.8 Hepatotoxicity
Hepatic failure, including fatal cases, has occurred in patients treated with REVLIMID in combination with dexamethasone. In clinical trials, 15% of patients
experienced hepatotoxicity (with hepatocellular, cholestatic and mixed characteristics); 2% of patients with MM and 1% of patients with myelodysplasia had serious
hepatotoxicity events. The mechanism of drug-induced hepatotoxicity is unknown. Pre-existing viral liver disease, elevated baseline liver enzymes, and concomitant
medications may be risk factors. Monitor liver enzymes periodically. Stop REVLIMID upon elevation of liver enzymes. After return to baseline values, treatment at a
lower dose may be considered.
5.9 Severe Cutaneous Reactions Including Hypersensitivity Reactions
Angioedema and severe cutaneous reactions including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and
systemic symptoms (DRESS) have been reported. DRESS may present with a cutaneous reaction (such as rash or exfoliative dermatitis), eosinophilia, fever, and/or
lymphadenopathy with systemic complications such as hepatitis, nephritis, pneumonitis, myocarditis, and/or pericarditis. These events can be fatal. Patients with a prior
history of Grade 4 rash associated with thalidomide treatment should not receive REVLIMID. REVLIMID interruption or discontinuation should be considered for
Grade 2-3 skin rash. REVLIMID must be discontinued for angioedema, Grade 4 rash, exfoliative or bullous rash, or if SJS, TEN or DRESS is suspected and should not
be resumed following discontinuation for these reactions.
5.10 Tumor Lysis Syndrome
Fatal instances of tumor lysis syndrome (TLS) have been reported during treatment with REVLIMID. The patients at risk of TLS a
re those with high tumor burden
prior to treatment. Monitor patients at risk closely and take appropriate preventive approaches. In the AUGMENT trial in FL or MZL patients, TLS occurred in 2
patients (1.1%) in the REVLIMID/rituximab arm. TLS occurred in 1 patient (0.5%) in the MAGNIFY trial during the REVLIMID/rituximab induction period; the
event was a serious, Grade 3 adverse reaction.
5.11 Tumor Flare Reaction
Tumor flare reaction (TFR) has occurred during investigational use of REVLIMID for CLL and lymphoma, and is characterized by tender lymph node swelling, low
grade fever, pain and rash. REVLIMID is not indicated and not recommended for use in CLL outside of controlled clinical trials.
Monitoring and evaluation for TFR is recommended in patients with MCL, FL, or MZL. Tumor flare reaction may mimic progression of disease (PD).
In the MCL trial, 13/134 (10%) of subjects experienced TFR; all reports were Grade 1 or 2 in severity. All of the events occurred in Cycle 1 and one patient developed
TFR again in Cycle 11. In the AUGMENT trial in FL or MZL patients, TFR was reported in 19/176 (10.8%) of patients in REVLIMID with rituximab arm; one patient
in the REVLIMID/rituximabarm experienced a Grade 3 TFR. In the MAGNIFY trial, 9/222 (4.1%) of patients experienced TFR; all reports were Grade 1 or 2 in
severity and 1 event was considered as serious.
REVLIMID may be continued in patients with Grade 1 and 2 TFR without interruption or modification, at the physician’s discretion. Patients with Grade 1 and 2 TFR
may also be treated with corticosteroids, non-steroidal anti-inflammatory drugs (NSAIDs) and/or narcotic analgesics for management of TFR symptoms. In patients
with Grade 3 or 4 TFR, it is recommended to withhold treatment with REVLIMID until TFR resolves to ≤ Grade 1. Patients with Grade 3 or 4 TFR may be treated for
management of symptoms per the guidance for treatment of Grade 1 and 2 TFR.
5.12 Impaired Stem Cell Mobilization
A decrease in the number of CD34+ cells collected after treatment (> 4 cycles) with REVLIMID has been reported. In patients who are auto-HSCT candidates, referral
to a transplant center should occur early in treatment to optimize the timing of the stem cell collection. In patients who received more than 4 cycles of a REVLIMID-
containing treatment or for whom inadequate numbers of CD 34+ cells have been collected with G-CSF alone, G-CSF with cyclophosphamide or the combination of G-
CSF with a CXCR4 inhibitor may be considered.
5.13 Thyroid Disorders
Both hypothyroidism and hyperthyroidism have been reported [see Adverse Reactions (6.2)]. Measure thyroid function before start of REVLIMID treatment and
during therapy.
5.14 Early Mortality in Patients with MCL
In another MCL study, there was an increase in early deaths (within 20 weeks), 12.9% in the REVLIMID arm versus 7.1% in the control arm. On exploratory
multivariate analysis, risk factors for early deaths include high tumor burden, MIPI score at diagnosis, and high WBC at baseline (≥ 10 x 10
9
/L).
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described in detail in other sections of the prescribing information:
o Embryo-Fetal Toxicity [see Boxed Warning, Warnings and Precautions (5.1, 5.2)]
10
Reference ID: 4439576
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
6.1
o Hematologic Toxicity [see Boxed Warning, Warnings and Precautions (5.3)]
o Venous and Arterial Thromboembolism [see Boxed Warning, Warnings and Precautions (5.4)]
o Increased Mortality in Patients with CLL [see Warnings and Precautions (5.5)]
o Second Primary Malignancies [see Warnings and Precautions (5.6)]
o Increased Mortality in Patients with MM When Pembrolizumab Is Added to a Thalidomide Analogue and Dexamethasone
[see Warnings and Precautions (5.7)]
o Hepatotoxicity [see Warnings and Precautions (5.8)]
o Severe Cutaneous Reactions Including Hypersensitivity Reactions [see Warnings and Precautions (5.9)]
o Tumor Lysis Syndrome [see Warnings and Precautions (5.10)]
o Tumor Flare Reactions [see Warnings and Precautions (5.11)]
o Impaired Stem Cell Mobilization [see Warnings and Precautions (5.12)]
o Thyroid Disorders [see Warnings and Precautions (5.13)]
o Early Mortality in Patients with MCL [see Warnings and Precautions (5.14)]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates
in the clinical trials of another drug and may not reflect the rates observed in practice.
Newly Diagnosed MM – REVLIMID Combination Therapy:
Data were evaluated from 1613 patients in a large phase 3 study who received at least one dose of REVLIMID with low dose dexamethasone (Rd) given for 2 different
durations of time (i.e., until progressive disease [Arm Rd Continuous; N=532] or for up to eighteen 28-day cycles [72 weeks, Arm Rd18; N=540] or who received
melphalan, prednisone and thalidomide (Arm MPT; N=541) for a maximum of twelve 42-day cycles (72 weeks). The median treatment duration in the Rd Continuous
arm was 80.2 weeks (range 0.7 to 246.7) or 18.4 months (range 0.16 to 56.7).
In general, the most frequently reported adverse reactions were comparable in Arm Rd Continuous and Arm Rd18, and included diarrhea, anemia, constipation,
peripheral edema, neutropenia, fatigue, back pain, nausea, asthenia, and insomnia. The most frequently reported Grade 3 or 4 reactions included neutropenia, anemia,
thrombocytopenia, pneumonia, asthenia, fatigue, back pain, hypokalemia, rash, cataract, lymphopenia, dyspnea, DVT, hyperglycemia, and leukopenia. The highest
frequency of infections occurred in Arm Rd Continuous (75%) compared to Arm MPT (56%). There were more grade 3 and 4 and serious adverse reactions of infection
in Arm Rd Continuous than either Arm MPT or Rd18.
In the Rd Continuous arm, the most common adverse reactions leading to dose interruption of REVLIMID were infection events (28.8%); overall, the median time to
the first dose interruption of REVLIMID was 7 weeks. The most common adverse reactions leading to dose reduction of REVLIMID in the Rd Continuous arm were
hematologic events (10.7%); overall, the median time to the first dose reduction of REVLIMID was 16 weeks. In the Rd Continuous arm, the most common adverse
reactions leading to discontinuation of REVLIMID were infection events (3.4%).
In both Rd arms, the frequencies of onset of adverse reactions were generally highest in the first 6 months of treatment and then the frequencies decreased over time or
remained stable throughout treatment, except for cataracts. The frequency of onset of cataracts increased over time with 0.7% during the first 6 months and up to 9.6%
by the 2nd year of treatment with Rd Continuous.
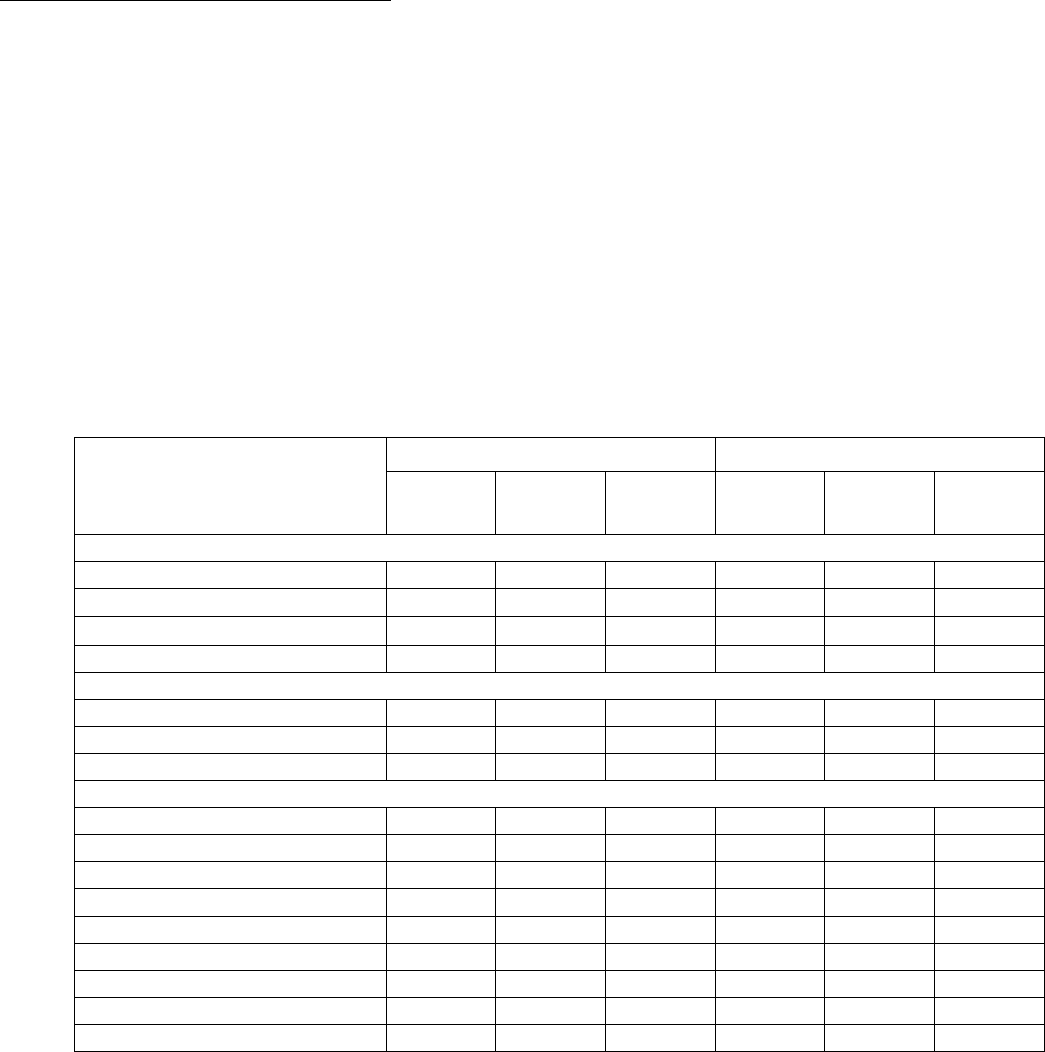
Table 4 summarizes the adverse reactions reported for the Rd Continuous, Rd18, and MPT treatment arms.
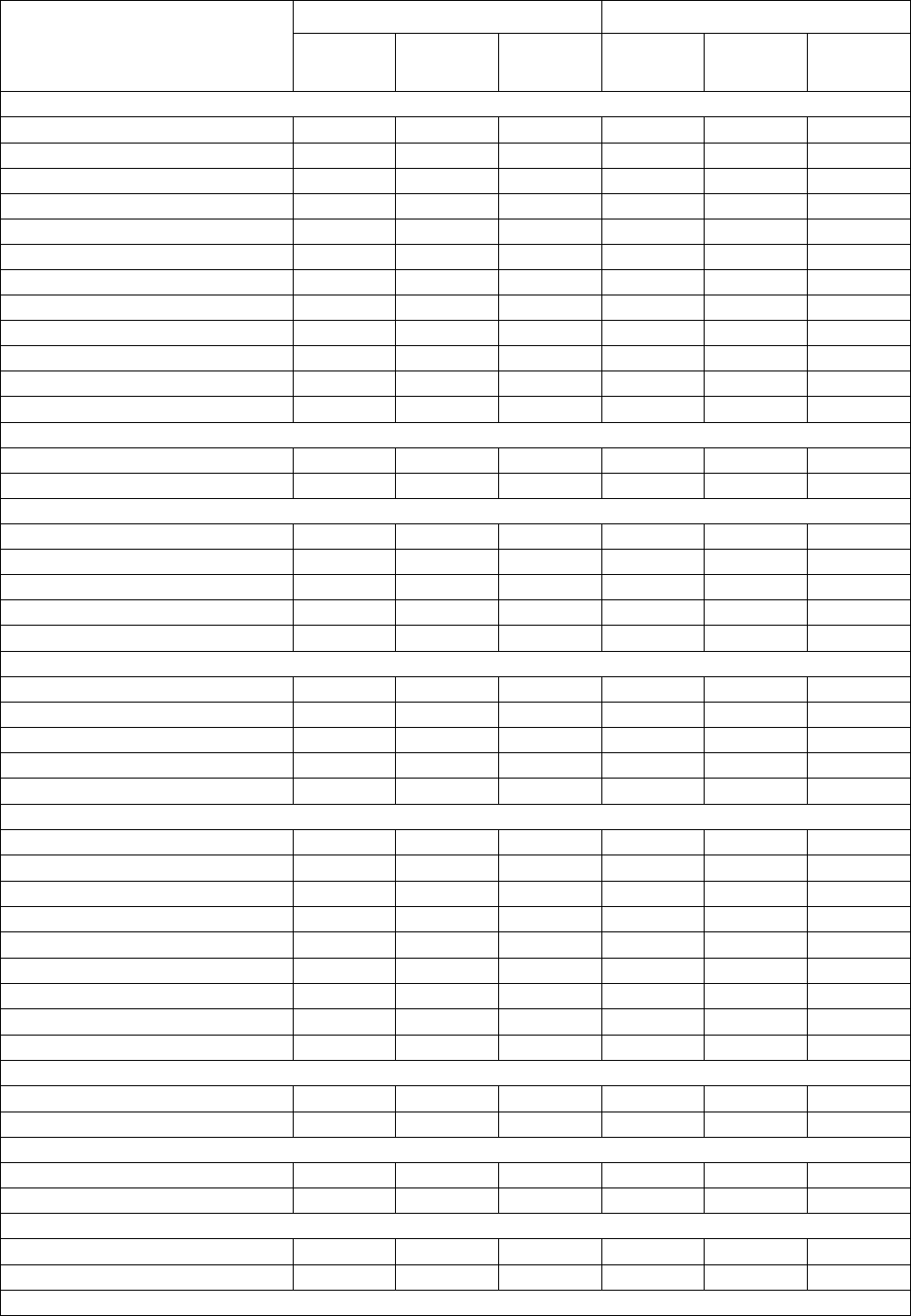
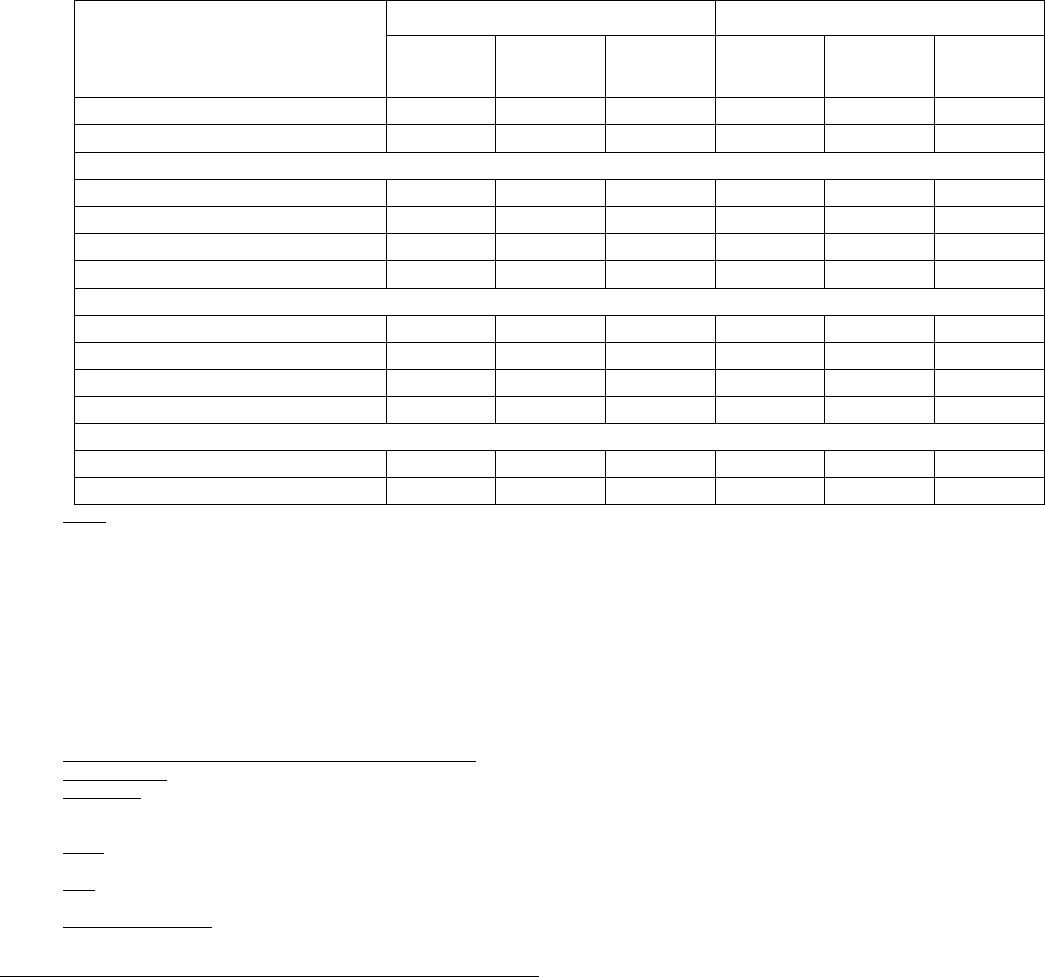
Table 4: All Adverse Reactions in ≥5% and Grade 3/4 Adverse Reactions in ≥1% of Patients with MM in the Rd Continuous or Rd18 Arms*
Body System
Adverse Reaction
All Adverse Reactions
a
Grade 3/4 Adverse Reactions
b
Rd
Continuous
(N = 532)
Rd18
(N = 540)
MPT
(N = 541)
Rd
Continuous
(N = 532)
Rd18
(N = 540)
MPT
(N = 541)
General disorders and administration site conditions
Fatigue
%
173 (33) 177 (33) 154 (28) 39 ( 7) 46 ( 9) 31 ( 6)
Asthenia 150 (28) 123 (23) 124 (23) 41 ( 8) 33 ( 6) 32 ( 6)
Pyrexia
c
114 (21) 102 (19) 76 (14)
13 ( 2) 7 ( 1) 7 ( 1)
Non-cardiac chest pain
f
29 ( 5) 31 ( 6) 18 ( 3) <1% < 1% < 1%
Gastrointestinal disorders
Diarrhea 242 (45) 208 (39) 89 (16) 21 ( 4) 18 ( 3) 8 ( 1)
Abdominal pain
% f
109 (20) 78 (14) 60 (11) 7 ( 1) 9 ( 2) < 1%
Dyspepsia
f
57 (11) 28 ( 5) 36 ( 7) <1% < 1% 0 ( 0)
Musculoskeletal and connective tissue disorders
Back pain
c
170 (32) 145 (27) 116 (21) 37 ( 7) 34 ( 6) 28 ( 5)
Muscle spasms
f
109 (20) 102 (19) 61 (11) < 1% < 1% < 1%
Arthralgia
f
101 (19) 71 (13) 66 (12) 9 ( 2) 8 ( 1) 8 ( 1)
Bone pain
f
87 (16) 77 (14) 62 (11) 16 ( 3) 15 ( 3) 14 ( 3)
Pain in extremity
f
79 (15) 66 (12) 61 (11) 8 ( 2) 8 ( 1) 7 ( 1)
Musculoskeletal pain
f
67 (13) 59 (11) 36 ( 7) < 1% < 1% < 1%
Musculoskeletal chest pain
f
60 (11) 51 ( 9) 39 ( 7) 6 ( 1) < 1% < 1%
Muscular weakness
f
43 ( 8) 35 ( 6) 29 ( 5) < 1% 8 ( 1) < 1%
Neck pain
f
40 ( 8) 19 ( 4) 10 ( 2) < 1% < 1% < 1%
Reference ID: 4439576
11
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Body System
Adverse Reaction
All Adverse Reactions
a
Grade 3/4 Adverse Reactions
b
Rd
Continuous
(N = 532)
Rd18
(N = 540)
MPT
(N = 541)
Rd
Continuous
(N = 532)
Rd18
(N = 540)
MPT
(N = 541)
Infections and infestations
Bronchitis
c
90 (17) 59 (11) 43 ( 8) 9 ( 2) 6 ( 1) < 1%
Nasopharyngitis
f
80 (15) 54 (10) 33 ( 6) 0 ( 0) 0 ( 0) 0 ( 0)
Urinary tract infection
f
76 (14) 63 (12) 41 ( 8) 8 ( 2) 8 ( 1) < 1%
Upper respiratory tract infection
c% f
69 (13) 53 ( 10) 31 ( 6) < 1% 8 ( 1) < 1%
Pneumonia
c@
93 (17) 87 (16) 56 (10) 60 (11) 57 (11) 41 ( 8)
Respiratory tract infection
%
35 ( 7) 25 ( 5) 21 ( 4) 7 ( 1) < 1% < 1%
Influenza
f
33 ( 6) 23 ( 4) 15 ( 3) < 1% < 1% 0 ( 0)
Gastroenteritis
f
32 ( 6) 17 ( 3) 13 ( 2) 0 ( 0) < 1% < 1%
Lower respiratory tract infection 29 ( 5) 14 ( 3) 16 ( 3) 10 ( 2) < 1% < 1%
Rhinitis
f
29 ( 5) 24 ( 4) 14 ( 3) 0 ( 0) 0 ( 0) 0 ( 0)
Cellulitis
c
< 5% < 5% < 5% 8 ( 2) < 1% < 1%
Sepsis
c@
33 ( 6) 26 ( 5) 18 ( 3) 26 ( 5) 20 ( 4) 13 ( 2)
Nervous system disorders
Headache
f
75 (14) 52 ( 10) 56 (10) < 1% < 1% < 1%
Dysgeusia
f
39 ( 7) 45 ( 8) 22 ( 4) < 1% 0 ( 0.0) < 1%
Blood and lymphatic system disorders
d
Anemia 233 (44) 193 (36) 229 (42) 97 (18) 85 (16) 102 (19)
Neutropenia 186 (35) 178 (33) 328 (61) 148 (28) 143 (26) 243 (45)
Thrombocytopenia 104 (20) 100 (19) 135 (25) 44 ( 8) 43 ( 8) 60 (11)
Febrile neutropenia 7 ( 1) 17 ( 3)
15 ( 3) 6 ( 1) 16 ( 3) 14 ( 3)
Pancytopenia < 1% 6 ( 1) 7 ( 1) < 1% < 1% < 1%
Respiratory, thoracic and mediastinal disorders
Cough
f
121 (23) 94 (17) 68 (13) < 1% < 1% < 1%
Dyspnea
c,e
117 (22) 89 (16) 113 (21)
30 ( 6) 22 ( 4) 18 ( 3)
Epistaxis
f
32 ( 6) 31 ( 6) 17 ( 3) < 1% < 1% 0 ( 0)
Oropharyngeal pain
f
30 ( 6) 22 ( 4) 14 ( 3) 0 ( 0) 0 ( 0) 0 ( 0)
Dyspnea exertional
e
27 ( 5) 29 ( 5) < 5%
6 ( 1) < 1% 0 ( 0)
Metabolism and nutrition disorders
Decreased appetite 123 (23) 115 (21) 72 (13)
14 ( 3) 7 ( 1) < 1%
Hypokalemia
%
91 (17) 62 (11) 38 ( 7) 35 ( 7) 20 ( 4) 11 ( 2)
Hyperglycemia 62 (12) 52 ( 10) 19 ( 4) 28 ( 5) 23 ( 4) 9 ( 2)
Hypocalcemia 57 (11) 56 (10) 31 ( 6)
23 ( 4) 19 ( 4) 8 ( 1)
Dehydration
%
25 ( 5) 29 ( 5) 17 ( 3) 8 ( 2) 13 ( 2) 9 ( 2)
Gou
t
e
< 5% < 5% < 5% 8 ( 2) 0 ( 0) 0 ( 0)
Diabetes mellitus
% e
< 5% < 5% < 5% 8 ( 2) < 1% < 1%
Hypophosphatemia
e
< 5% < 5% < 5%
7 ( 1) < 1% < 1%
Hyponatremia
% e
< 5% < 5% < 5% 7 ( 1) 13 ( 2) 6 ( 1)
Skin and subcutaneous tissue disorders
Rash 139 (26) 151 (28) 105 (19) 39 ( 7) 38 ( 7) 33 ( 6)
Pruritus
f
47 ( 9) 49 ( 9) 24 ( 4) < 1% < 1% < 1%
Psychiatric disorders
Insomnia 147 (28) 127 (24) 53 ( 10) < 1% 6 ( 1) 0 ( 0)
Depression 58 (11) 46 ( 9) 30 ( 6) 10 ( 2) < 1% < 1%
Vascular disorders
Deep vein thrombosis
c%
55 (10) 39 ( 7) 22 ( 4)
30 ( 6) 20 ( 4) 15 ( 3)
Hypotension
c%
51 ( 10) 35 ( 6) 36 ( 7)
11 ( 2) 8 ( 1) 6 ( 1)
Injury, Poisoning, and Procedural Complications
Reference ID: 4439576
12
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Body System
Adverse Reaction
All Adverse Reactions
a
Grade 3/4 Adverse Reactions
b
Rd
Continuous
(N = 532)
Rd18
(N = 540)
MPT
(N = 541)
Rd
Continuous
(N = 532)
Rd18
(N = 540)
MPT
(N = 541)
Fall
f
43 ( 8) 25 ( 5) 25 ( 5) < 1% 6 ( 1) 6 ( 1)
Contusion
f
33 ( 6) 24 ( 4) 15 ( 3) < 1% < 1% 0 ( 0)
Eye disorders
Cataract 73 (14) 31 ( 6) < 1% 31 ( 6) 14 ( 3) < 1%
Cataract subcapsula
r
e
< 5% < 5% < 5% 7 ( 1) 0 ( 0) 0 ( 0)
Investigations
Weight decreased 72 (14) 78 (14) 48 ( 9) 11 ( 2) < 1% < 1%
Cardiac disorders
Atrial fibrillation
c
37 ( 7) 25 ( 5) 25 ( 5) 13 ( 2) 9 ( 2) 6 ( 1)
Myocardial infarction (including acute)
c ,e
< 5% < 5% < 5% 10 ( 2) < 1% < 1%
Renal and Urinary disorders
Renal failure (including acute)
c@,f
49 ( 9) 54 (10) 37 ( 7) 28 ( 5) 33 ( 6) 29 ( 5)
Neoplasms benign, malignant and unspecified (Including cysts and polyps)
Squamous cell carcinoma
c e
< 5% < 5% < 5% 8 ( 2) < 1% 0 ( 0)
Basal cell carcinoma
c e,f
< 5% < 5% < 5% < 1% < 1% 0 ( 0)
Note: A subject with multiple occurrences of an adverse reaction is counted only once under the applicable Body System/Adverse Reaction.
a
All treatment-emergent adverse events in at least 5% of subjects in the Rd Continuous or Rd18 Arms and at least a 2% higher frequency (%) in either the Rd
Continuous or Rd18 Arms compared to the MPT Arm.
b
All grade 3 or 4 treatment-emergent adverse events in at least 1% of subjects in the Rd Continuous or Rd18 Arms and at least a 1% higher frequency (%) in
either the Rd Continuous or Rd18 Arms compared to the MPT Arm.
c
Serious treatment-emergent adverse events in at least 1% of subjects in the Rd Continuous or Rd18 Arms and at least a 1% higher frequency (%) in either
the Rd Continuous or Rd18 Arms compared to the MPT Arm.
d
Preferred terms for the blood and lymphatic system disorders body system were included by medical judgment as known adverse reactions for
Rd Continuous/Rd18, and have also been reported as serious.
e
Footnote “a” not applicable.
f
Footnote “b” not applicable.
@
- adverse reactions in which at least one resulted in a fatal outcome.
%
- adverse reactions in which at least one was considered to be life threatening (if the outcome of the reaction was death, it is included with death cases).
*Adverse reactions included in combined adverse reaction terms:
Abdominal Pain: Abdominal pain, abdominal pain upper, abdominal pain lower, gastrointestinal pain
Pneumonias: Pneumonia, lobar pneumonia, pneumonia pneumococcal, bronchopneumonia, pneumocystis jiroveci pneumonia, pneumonia legionella,
pneumonia staphylococcal, pneumonia klebsiella, atypical pneumonia, pneumonia bacterial, pneumonia escherichia, pneumonia streptococcal, pneumonia
viral
Sepsis: Sepsis, septic shock, urosepsis, escherichia sepsis, neutropenic sepsis, pneumococcal sepsis, staphylococcal sepsis, bacterial sepsis, meningococcal
sepsis, enterococcal sepsis, klebsiella sepsis, pseudomonal sepsis
Rash: Rash, rash pruritic, rash erythematous, rash maculo-papular, rash generalized, rash papular, exfoliative rash, rash follicular, rash macular, drug rash
with eosinophilia and systemic symptoms, erythema multiforme, rash pustular
Deep Vein Thrombosis: Deep vein thrombosis, venous thrombosis limb, venous thrombosis
Newly Diagnosed MM - REVLIMID Maintenance Therapy Following Auto-HSCT:
Data were evaluated from 1018 patients in two randomized trials who received at least one dose of REVLIMID 10 mg daily as maintenance therapy after auto-HSCT
until progressive disease or unacceptable toxicity. The mean treatment duration for REVLIMID treatment was 30.3 months for Maintenance Study 1 and 24.0 months
for Maintenance Study 2 (overall range across both studies from 0.1 to 108 months). As of the cut-off date of 1 Mar 2015, 48 patients (21%) in the Maintenance Study
1 REVLIMID arm were still on treatment and none of the patients in the Maintenance Study 2 REVLIMID arm were still on treatment at the same cut-off date
The adverse reactions listed from Maintenance Study 1 included events reported post-transplant (completion of high-dose melphalan /auto-HSCT), and the
maintenance treatment period. In Maintenance Study 2, the adverse reactions were from the maintenance treatment period only. In general, the most frequently reported
adverse reactions (more than 20% in the REVLIMID arm) across both studies were neutropenia, thrombocytopenia, leukopenia, anemia, upper respiratory tract
infection, bronchitis, nasopharyngitis, cough, gastroenteritis, diarrhea, rash, fatigue, asthenia, muscle spasm and pyrexia. The most frequently reported Grade 3 or 4
reactions (more than 20% in the REVLIMID arm) included neutropenia, thrombocytopenia, and leukopenia. The serious adverse reactions lung infection and
neutropenia (more than 4.5%) occurred in the REVLIMID arm.
For REVLIMID, the most common adverse reactions leading to dose interruption were hematologic events (29.7%, data available in Maintenance Study 2 only). The
most common adverse reaction leading to dose reduction of REVLIMID were hematologic events (17.7%, data available in Maintenance Study 2 only). The most
common adverse reactions leading to discontinuation of REVLIMID were thrombocytopenia (2.7%) in Maintenance Study 1 and neutropenia (2.4%) in Maintenance
Study 2.
The frequencies of onset of adverse reactions were generally highest in the first 6 months of treatment and then the frequencies decreased over time or remained stable
throughout treatment.
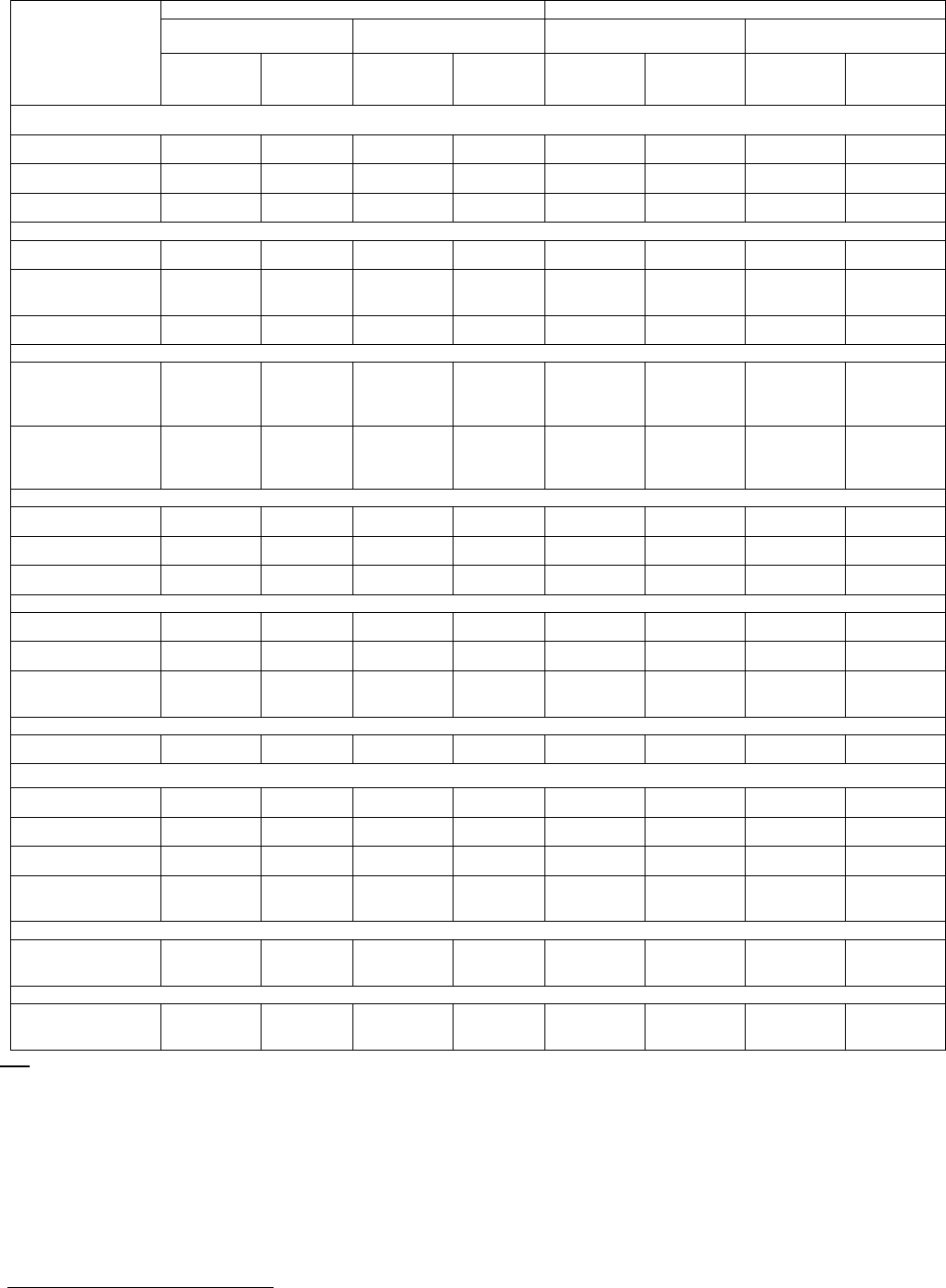
Table 5 summarizes the adverse reactions reported for the REVLIMID and placebo maintenance treatment arms.
Reference ID: 4439576
13
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
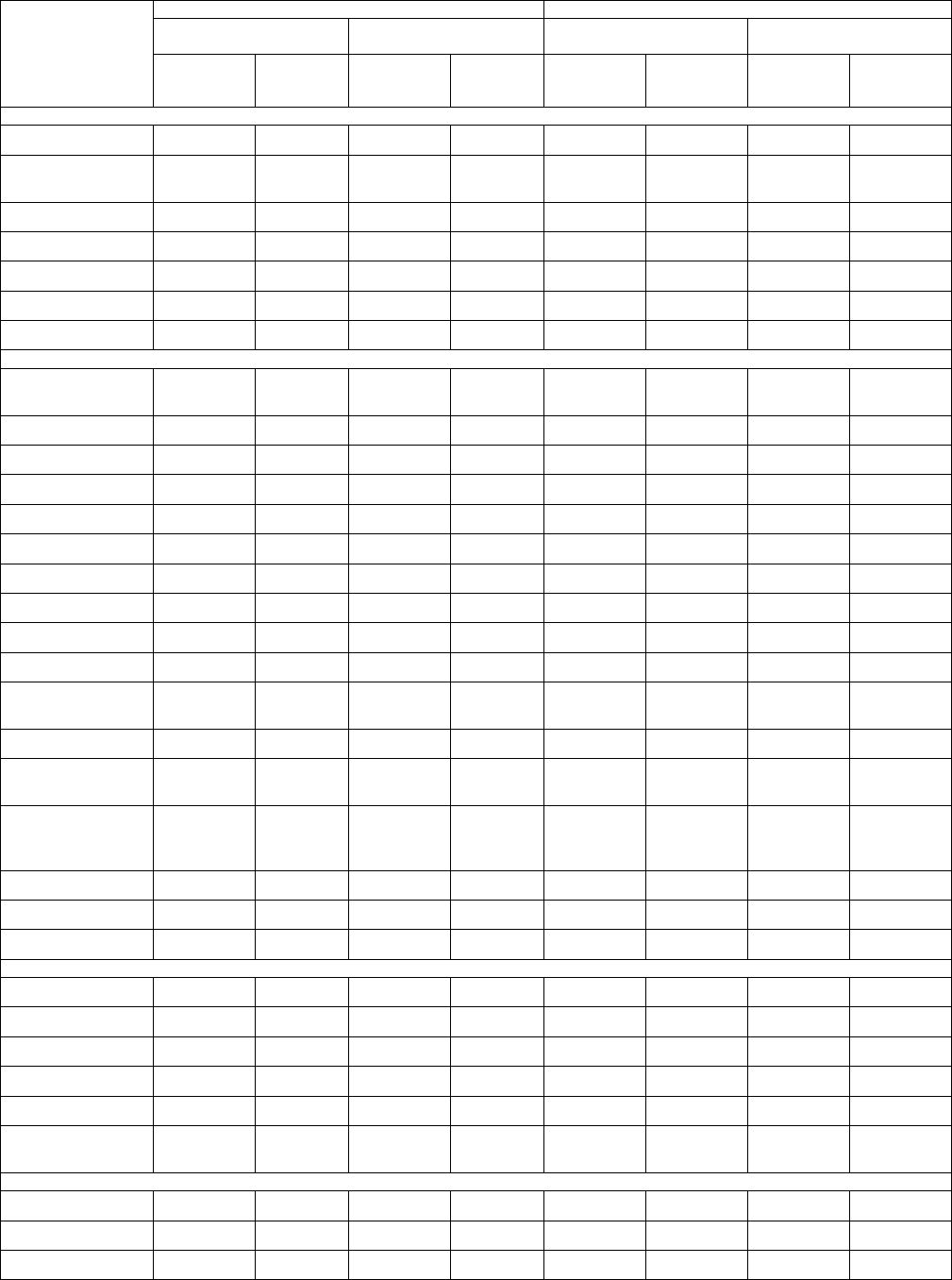
Table 5: All Adverse Reactions in ≥5% and Grade 3/4 Adverse Reactions in ≥1% of Patients with MM in the REVLIMID Vs Placebo Arms*
Body System
Adverse Reaction
Maintenance Study 1 Maintenance Study 2
All Adverse Reactions
a
Grade 3/4 Adverse
Reactions
b
All Adverse Reactions
a
Grade 3/4 Adverse
Reactions
b
REVLIMID
(N=224)
n (%)
Placebo
(N=221)
n (%)
REVLIMID
(N=224)
n (%)
Placebo
(N=221)
n (%)
REVLIMID
(N=293)
n (%)
Placebo
(N=280)
n (%)
REVLIMID
(N=293)
n (%)
Placebo
(N=280)
n (%)
Blood and lymphatic system disorders
Neutropenia
c %
177 ( 79) 94 ( 43) 133 ( 59) 73 ( 33) 178 ( 61) 33 ( 12) 158 ( 54) 21 ( 8)
Thrombocytopenia
c
%
162 ( 72) 101 ( 46) 84 ( 38) 67 ( 30) 69 ( 24) 29 ( 10) 38 ( 13) 8 ( 3)
Leukopenia
c
51 ( 23) 25 ( 11) 45 ( 20) 22 ( 10) 93 ( 32) 21 ( 8) 71 ( 24) 5 ( 2)
Anemia 47 ( 21) 27 ( 12) 23 ( 10) 18 ( 8) 26 ( 9) 15 ( 5) 11 ( 4) 3 ( 1)
Lymphopenia 40 ( 18) 29 ( 13) 37 ( 17) 26 ( 12) 13 ( 4) 3 ( 1) 11 ( 4) < 1%
Pancytopenia
c d %
< 1% 0 ( 0) 0 ( 0) 0 ( 0) 12 ( 4) < 1% 7 ( 2) < 1%
Febrile neutropenia
c
39 ( 17) 34 ( 15) 39 ( 17) 34 ( 15) 7 ( 2) < 1% 5 ( 2) < 1%
Infections and infestations
#
Upper respiratory
tract infection
e
60 ( 27) 35 ( 16) 7 ( 3) 9 ( 4) 32 ( 11) 18 ( 6) < 1% 0 ( 0)
Neutropenic infection 40 ( 18) 19 ( 9) 27 ( 12) 14 ( 6) 0 ( 0) 0 ( 0) 0 ( 0) 0 ( 0)
Pneumonias*
c %
31 ( 14) 15 ( 7) 23 ( 10) 7 ( 3) 50 ( 17) 13 ( 5) 27 ( 9) 5 ( 2)
Bronchitis
c
10 ( 4) 9 ( 4) < 1% 5 ( 2) 139 ( 47) 104 ( 37) 4 ( 1) < 1%
Nasopharyngitis
e
5 ( 2) < 1% 0 ( 0) 0 ( 0) 102 ( 35) 84 ( 30) < 1% 0 ( 0)
Gastroenteritis
c
0 ( 0) 0 ( 0) 0 ( 0) 0 ( 0) 66 ( 23) 55 ( 20) 6 ( 2) 0 ( 0)
Rhinitis
e
< 1% 0 ( 0) 0 ( 0) 0 ( 0) 44 ( 15) 19 ( 7) 0 ( 0) 0 ( 0)
Sinusitis
e
8 ( 4) 3 ( 1) 0 ( 0) 0 ( 0) 41 ( 14) 26 ( 9) 0 ( 0) < 1%
Influenza
c
8 ( 4) 5 ( 2) < 1% < 1% 39 ( 13) 19 ( 7) 3 ( 1) 0 ( 0)
Lung infection
c
21 ( 9) < 1% 19 ( 8) < 1% 9 ( 3) 4 ( 1) < 1% 0 ( 0)
Lower respiratory
tract infection
e
13 ( 6) 5 ( 2) 6 ( 3) 4 ( 2) 4 ( 1) 4 ( 1) 0 ( 0) < 1%
Infection
c
12 ( 5) 6 ( 3) 9 ( 4) 5 ( 2) 17 ( 6) 5 ( 2) 0 ( 0) 0 ( 0)
Urinary tract
infection
c d e
9 ( 4) 5 ( 2) 4 ( 2) 4 ( 2)
22 ( 8) 17 ( 6) < 1% 0 ( 0)
Lower respiratory
tract infection
bacterial
d
6 ( 3) < 1% 4 ( 2) 0 ( 0) 0 ( 0) 0 ( 0) 0 ( 0) 0 ( 0)
Bacteremia
d
5 ( 2) 0 ( 0) 4 ( 2) 0 ( 0) 0 ( 0) 0 ( 0) 0 ( 0) 0 ( 0)
Herpes zoster
c d
11 ( 5) 10 ( 5) 3 ( 1) < 1% 29 ( 10) 25 ( 9) 6 ( 2) < 1%
Sepsis*
c d @
< 1% < 1% 0 ( 0) 0 ( 0) 6 ( 2) < 1% 4 ( 1) < 1%
Gastrointestinal disorders
Diarrhea 122 ( 54) 83 ( 38) 22 ( 10) 17 ( 8) 114 ( 39) 34 ( 12) 7 ( 2) 0 ( 0)
Nausea
e
33 ( 15) 22 ( 10) 16 ( 7) 10 ( 5) 31 ( 11) 28 ( 10) 0 ( 0) 0 ( 0)
Vomiting 17 ( 8) 12 ( 5) 8 ( 4) 5 ( 2) 16 ( 5) 15 ( 5) < 1% 0 ( 0)
Constipation
e
12 ( 5) 8 ( 4) 0 ( 0) 0 ( 0) 37 ( 13) 25 ( 9) < 1% 0 ( 0)
Abdominal pain
e
8 ( 4) 7 ( 3) < 1% 4 ( 2) 31 ( 11) 15 ( 5) < 1% < 1%
Abdominal pain
upper
e
0 ( 0) 0 ( 0) 0 ( 0) 0 ( 0) 20 ( 7) 12 ( 4) < 1% 0 ( 0)
General disorders and administration site conditions
Asthenia 0 ( 0) < 1% 0 ( 0) 0 ( 0) 87 ( 30) 53 ( 19) 10 ( 3) < 1%
Fatigue 51 ( 23) 30 ( 14) 21 ( 9) 9 ( 4) 31 ( 11) 15 ( 5) 3 ( 1) 0 ( 0)
Pyrexia
e
17 ( 8) 10 ( 5) < 1% < 1% 60 ( 20) 26 ( 9) < 1% 0 ( 0)
Reference ID: 4439576
14
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Body System
Adverse Reaction
Maintenance Study 1 Maintenance Study 2
All Adverse Reactions
a
Grade 3/4 Adverse
Reactions
b
All Adverse Reactions
a
Grade 3/4 Adverse
Reactions
b
REVLIMID
(N=224)
n (%)
Placebo
(N=221)
n (%)
REVLIMID
(N=224)
n (%)
Placebo
(N=221)
n (%)
REVLIMID
(N=293)
n (%)
Placebo
(N=280)
n (%)
REVLIMID
(N=293)
n (%)
Placebo
(N=280)
n (%)
Skin and subcutaneous tissue disorders
Dry skin
e
9 ( 4) 4 ( 2) 0 ( 0) 0 ( 0) 31 ( 11) 21 ( 8) 0 ( 0) 0 ( 0)
Rash 71 ( 32) 48 ( 22) 11 ( 5) 5 ( 2) 22 ( 8) 17 ( 6) 3 ( 1) 0 ( 0)
Pruritus 9 ( 4) 4 ( 2) 3 ( 1) 0 ( 0) 21 ( 7) 25 ( 9) < 1% 0 ( 0)
Nervous system disorders
Paresthesia
e
< 1% 0 ( 0) 0 ( 0) 0 ( 0) 39 ( 13) 30 ( 11) < 1% 0 ( 0)
Peripheral
neuropathy*
e
34 ( 15) 30 ( 14) 8 ( 4) 8 ( 4) 29 ( 10) 15 ( 5) 4 ( 1) < 1%
Headache
d
11 ( 5) 8 ( 4) 5 ( 2) < 1% 25 ( 9) 21 ( 8) 0 ( 0) 0 ( 0)
Investigations
Alanine
aminotransferase
increased
16 ( 7) 3 ( 1) 8 ( 4) 0 ( 0) 5 ( 2) 5 ( 2) 0 ( 0) < 1%
Aspartate
aminotransferase
increased
d
13 ( 6) 5 ( 2) 6 ( 3) 0 ( 0) < 1% 5 ( 2) 0 ( 0) 0 ( 0)
Metabolism and nutrition disorders
Hypokalemia 24 ( 11) 13 ( 6) 16 ( 7) 12 ( 5) 12 ( 4) < 1% < 1% 0 ( 0)
Dehydration 9 ( 4 ) 5 ( 2) 7 ( 3) 3 ( 1) 0 ( 0) 0 ( 0) 0 ( 0) 0 ( 0)
Hypophosphatemia
d
16 ( 7) 15 ( 7) 13 ( 6) 14 ( 6) 0 ( 0) < 1% 0 ( 0) 0 ( 0)
Musculoskeletal and connective tissue disorders
Muscle spasms
e
0 ( 0) < 1% 0 ( 0) 0 ( 0) 98 ( 33) 43 ( 15) < 1% 0 ( 0)
Myalgia
e
7 ( 3) 8 ( 4) 3 ( 1) 5 ( 2) 19 ( 6) 12 ( 4) < 1% < 1%
Musculoskeletal pain
e
< 1% < 1% 0 ( 0) 0 ( 0) 19 ( 6) 11 ( 44) 0 ( 0) 0 ( 0)
Hepatobiliary disorders
Hyperbilirubinemia
e
34 ( 15) 19 ( 9) 4 ( 2) < 1% 4 ( 1) < 1% < 1% 0 ( 0)
Respiratory, thoracic and mediastinal disorders
Cough
e
23 ( 10) 12 ( 5) 3 ( 1) < 1% 80 ( 27) 56 ( 20) 0 ( 0) 0 ( 0)
Dyspnea
c e
15 ( 7) 9 ( 4) 8 ( 4) 4 ( 2) 17 ( 6) 9 ( 3) < 1% 0 ( 0)
Rhinorrhea
e
0 ( 0) 3 ( 1) 0 ( 0) 0 ( 0) 15 ( 5) 6 ( 2) 0 ( 0) 0 ( 0)
Pulmonary embolism
c d e
0 ( 0) 0 ( 0) 0 ( 0) 0 ( 0) 3 ( 1) 0 ( 0) < 1% 0 ( 0)
Vascular disorders
Deep vein
thrombosis*
c d %
8 ( 4) < 1% 5 ( 2) < 1% 7 ( 2) < 1% 4 ( 1) < 1%
Neoplasms benign, malignant and unspecified (including cysts and polyps)
Myelodysplastic
syndrome
c d e
5 ( 2) 0 ( 0) < 1% 0 ( 0) 3 ( 1) 0 ( 0) < 1% 0 ( 0)
Note: Adverse Events (AEs) are coded to Body System /Adverse Reaction using MedDRA v15.1. A subject with multiple occurrences of an adverse reaction is
counted only once under the applicable Body System/Adverse Reaction.
a
All treatment-emergent AEs in at least 5% of patients in the REVLIMID Maintenance group and at least 2% higher frequency (%) than the Placebo Maintenance
group.
b
All grade 3 or 4 treatment-emergent AEs in at least 1% of patients in the REVLIMID Maintenance group and at least 1% higher frequency (%) than the Placebo
Maintenance group.
c
All serious treatment-emergent AEs in at least 1% of patients in the REVLIMID Maintenance group and at least 1% higher frequency (%) than the Placebo
Maintenance group.
d
Footnote “a” not applicable for either study
e
Footnote “b” not applicable for either study
@
-ADRs where at least one resulted in a fatal outcome
%
- ADRs where at least one was considered to be Life Threatening (if the outcome of the event was death, it is included with death cases)
# - All adverse reactions under Body System of Infections and Infestation except for rare infections of Public Health interest will be considered listed
*Adverse Reactions for combined ADR terms (based on relevant TEAE PTs included in Maintenance Studies 1 and 2 [per MedDRA v 15.1]):
Reference ID: 4439576
15
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Pneumonias Bronchopneumonia, Lobar pneumonia, Pneumocystis jiroveci pneumonia, Pneumonia, Pneumonia klebsiella, Pneumonia legionella, Pneumonia
mycoplasmal, Pneumonia pneumococcal, Pneumonia streptococcal, Pneumonia viral, Lung disorder, Pneumonitis
Sepsis: Bacterial sepsis, Pneumococcal sepsis, Sepsis, Septic shock, Staphylococcal sepsis
Peripheral neuropathy: Neuropathy peripheral, Peripheral motor neuropathy, Peripheral sensory neuropathy, Polyneuropathy
Deep vein thrombosis: Deep vein thrombosis, Thrombosis, Venous thrombosis
After At Least One Prior Therapy for MM:
Data were evaluated from 703 patients in two studies who received at least one dose of REVLIMID/dexamethasone (353 patients) or placebo/dexamethasone (350
patients).
In the REVLIMID/dexamethasone treatment group, 269 patients (76%) had at least one dose interruption with or without a dose reduction of REVLIMID compared to
199 patients (57%) in the placebo/dexamethasone treatment group. Of these patients who had one dose interruption with or without a dose reduction, 50% in the
REVLIMID/dexamethasone treatment group had at least one additional dose interruption with or without a dose reduction compared to 21% in the
placebo/dexamethasone treatment group. Most adverse reactions and Grade 3/4 adverse reactions were more frequent in patients who received the combination of
REVLIMID/dexamethasone compared to placebo/dexamethasone.
Tables 6, 7, and 8 summarize the adverse reactions reported for REVLIMID/dexamethasone and placebo/dexamethasone groups.
Table 6: Adverse Reactions Reported in ≥5% of Patients and with a ≥2% Difference in Proportion of Patients with MM between the
REVLIMID/dexamethasone and Placebo/dexamethasone Groups
Body System
Adverse Reaction
REVLIMID/Dex
(N=353)
n (%)
Placebo/Dex
(N=350)
n (%)
Blood and lymphatic system disorders
Neutropenia
%
149 (42) 22 ( 6)
Anemia
@
111 (31) 83 (24)
Thrombocytopenia
@
76 (22) 37 (11)
Leukopenia 28 ( 8) 4 ( 1)
Lymphopenia 19 ( 5) 5 ( 1)
General disorders and administration site conditions
Fatigue 155 (44) 146 (42)
Pyrexia 97 (27) 82 (23)
Peripheral edema 93 (26) 74 (21)
Chest pain 29 ( 8) 20 ( 6)
Lethargy 24 ( 7) 8 ( 2)
Gastrointestinal disorders
Constipation 143 (41) 74 (21)
Diarrhea
@
136 (39) 96 (27)
Nausea
@
92 (26) 75 (21)
Vomiting
@
43 (12) 33 ( 9)
Abdominal pain
@
35 ( 10) 22 ( 6)
Dry mouth 25 ( 7) 13 ( 4)
Musculoskeletal and connective tissue disorders
Muscle cramp 118 (33) 74 (21)
Back pain 91 (26) 65 (19)
Bone pain 48 (14) 39 (11)
Pain in limb 42 (12) 32 ( 9)
Nervous system disorders
Dizziness 82 (23) 59 (17)
Tremor 75 (21) 26 ( 7)
Dysgeusia 54 (15) 34 ( 10)
Hypoesthesia 36 (10) 25 ( 7)
Neuropathyª 23 ( 7) 13 ( 4)
Respiratory, thoracic and mediastinal disorders
Dyspnea 83 (24) 60 (17)
Nasopharyngitis 62 (18) 31 ( 9)
Pharyngitis 48 (14) 33 ( 9)
Bronchitis 40 (11) 30 ( 9)
Infections
b
and infestations
Reference ID: 4439576
16
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Body System
Adverse Reaction
REVLIMID/Dex
(N=353)
n (%)
Placebo/Dex
(N=350)
n (%)
Upper respiratory tract infection 87 (25) 55 (16)
Pneumonia
@
48 (14) 29 ( 8)
Urinary tract infection 30 ( 8) 19 ( 5)
Sinusitis 26 ( 7) 16 ( 5)
Skin and subcutaneous system disorders
Rash
c
75 (21) 33 ( 9)
Sweating increased 35 ( 10) 25 ( 7)
Dry skin 33 ( 9) 14 ( 4)
Pruritus 27 ( 8) 18 ( 5)
Metabolism and nutrition disorders
Anorexia 55 (16) 34 ( 10)
Hypokalemia 48 (14) 21 ( 6)
Hypocalcemia 31 ( 9) 10 ( 3)
Appetite decreased 24 ( 7) 14 ( 4)
Dehydration 23 ( 7) 15 ( 4)
Hypomagnesemia 24 ( 7) 10 ( 3)
Investigations
Weight decreased 69 (20) 52 (15)
Eye disorders
Blurred vision 61 (17) 40 (11)
Vascular disorders
Deep vein thrombosis
%
33 ( 9) 15 ( 4)
Hypertension 28 ( 8) 20 ( 6)
Hypotension 25 ( 7) 15 ( 4)
Table 7: Grade 3/4 Adverse Reactions Reported in ≥2% Patients and with a ≥1% Difference in Proportion of Patients with MM between the
REVLIMID/dexamethasone and Placebo/dexamethasone groups
Body System
Adverse Reaction
REVLIMID/Dex
(N=353)
n (%)
Placebo/Dex
(N=350)
n (%)
Blood and lymphatic system disorders
Neutropenia
%
118 (33) 12 ( 3)
Thrombocytopenia
@
43 (12) 22 ( 6)
Anemia
@
35 ( 10) 20 ( 6)
Leukopenia 14 ( 4) < 1%
Lymphopenia 10 ( 3) 4 ( 1)
Febrile neutropenia
%
8 ( 2) 0 ( 0)
General disorders and administration site conditions
Fatigue 23 ( 7) 17 ( 5)
Vascular disorders
Deep vein thrombosis
%
29 ( 8) 12 ( 3)
Infections and infestations
Pneumonia
@
30 ( 8) 19 ( 5)
Urinary tract infection 5 ( 1) < 1%
Metabolism and nutrition disorders
Hypokalemia 17 ( 5) 5 ( 1)
Hypocalcemia 13 ( 4) 6 ( 2)
Hypophosphatemia 9 ( 3) 0 ( 0)
Respiratory, thoracic and mediastinal disorders
Pulmonary embolism
@
14 ( 4) < 1%
Respiratory distress
@
4 ( 1) 0 ( 0)
Reference ID: 4439576
17
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Body System
Adverse Reaction
REVLIMID/Dex
(N=353)
n (%)
Placebo/Dex
(N=350)
n (%)
Musculoskeletal and connective tissue disorders
Muscle weakness 20 ( 6) 10 ( 3)
Gastrointestinal disorders
Diarrhea
@
11 ( 3) 4 ( 1)
Constipation 7 ( 2) < 1%
Nausea
@
6 ( 2) < 1%
Cardiac disorders
Atrial fibrillation
@
13 ( 4) 4 ( 1)
Tachycardia 6 ( 2) < 1%
Cardiac failure congestive
@
5 ( 1) < 1%
Nervous system disorders
Syncope 10 ( 3) < 1%
Dizziness 7 ( 2) < 1%
Eye disorders
Cataract 6 ( 2) < 1%
Cataract unilateral 5 ( 1) 0 ( 0)
Psychiatric disorder
Depression 10 ( 3) 6 ( 2)
Table 8: Serious Adverse Reactions Reported in ≥1% Patients and with a ≥1% Difference in Proportion of Patients with MM between the
REVLIMID/dexamethasone and Placebo/dexamethasone Groups
Body System
Adverse Reaction
REVLIMID/Dex
(N=353)
n (%)
Placebo/Dex
(N=350)
n (%)
Blood and lymphatic system disorders
Febrile neutropenia
%
6 ( 2) 0 ( 0)
Vascular disorders
Deep vein thrombosis
%
26 ( 7) 11 ( 3)
Infections and infestations
Pneumonia
@
33 ( 9) 21 ( 6)
Respiratory, thoracic, and mediastinal disorders
Pulmonary embolism
@
13 ( 4) < 1%
Cardiac disorders
Atrial fibrillation
@
11 ( 3) < 1%
Cardiac failure congestive
@
5 ( 1) 0 ( 0)
Nervous system disorders
Cerebrovascular accident
@
7 ( 2) < 1%
Gastrointestinal disorders
Diarrhea
@
6 ( 2) < 1%
Musculoskeletal and connective tissue disorders
Bone pain 4 ( 1) 0 ( 0)
For Tables 6, 7 and 8 above:
@
- adverse reactions in which at least one resulted in a fatal outcome.
%
- adverse reactions in which at least one was considered to be life threatening (if the outcome of the reaction was death, it is included with death cases).
Median duration of exposure among patients treated with REVLIMID/dexamethasone was 44 weeks while median duration of exposure among patients treated with
placebo/dexamethasone was 23 weeks. This should be taken into consideration when comparing frequency of adverse reactions between two treatment groups
REVLIMID/dexamethasone vs. placebo/dexamethasone.
Venous and Arterial Thromboembolism [see Boxed Warning, Warnings and Precautions (5.4)]
VTE and ATE are increased in patients treated with REVLIMID.
Deep vein thrombosis (DVT) was reported as a serious (7.4%) or severe (8.2%) adverse drug reaction at a higher rate in the REVLIMID/dexamethasone group
compared to 3.1 % and 3.4% in the placebo/dexamethasone group, respectively in the 2 studies in patients with at least 1 prior therapy with discontinuations due to
DVT adverse reactions reported at comparable rates between groups. In the NDMM study, DVT was reported as an adverse reaction (all grades: 10.3%, 7.2%, 4.1%),
Reference ID: 4439576
18
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
as a serious adverse reaction (3.6%, 2.0%, 1.7%), and as a Grade 3/4 adverse reaction (5.6%, 3.7%, 2.8%) in the Rd Continuous, Rd18, and MPT Arms, respectively.
Discontinuations and dose reductions due to DVT adverse reactions were reported at comparable rates between the Rd Continuous and Rd18 Arms (both <1%).
Interruption of REVLIMID treatment due to DVT adverse reactions was reported at comparable rates between the Rd Continuous (2.3%) and Rd18 (1.5%) arms.
Pulmonary embolism (PE) was reported as a serious adverse drug reaction (3.7%) or Grade 3/4 (4.0%) at a higher rate in the REVLIMID/dexamethasone group
compared to 0.9% (serious or grade 3/4) in the placebo/dexamethasone group in the 2 studies in patients with, at least 1 prior therapy, with discontinuations due to PE
adverse reactions reported at comparable rates between groups. In the NDMM study, the frequency of adverse reactions of PE was similar between the Rd Continuous,
Rd18, and MPT Arms for adverse reactions (all grades: 3.9%, 3.3%, and 4.3%, respectively), serious adverse reactions (3.8%, 2.8%, and 3.7%, respectively), and grade
3/4 adverse reactions (3.8%, 3.0%, and 3.7%, respectively).
Myocardial infarction was reported as a serious (1.7%) or severe (1.7%) adverse drug reaction at a higher rate in the REVLIMID/dexamethasone group compared to 0.6
% and 0.6% respectively in the placebo/dexamethasone group. Discontinuation due to MI (including acute) adverse reactions was 0.8% in REVLIMID/dexamethasone
group and none in the placebo/dexamethasone group. In the NDMM study, myocardial infarction (including acute) was reported as an adverse reaction (all grades:
2.4%, 0.6%, and 1.1%), as a serious adverse reaction, (2.3%, 0.6%, and 1.1%), or as a severe adverse reaction (1.9%, 0.6%, and 0.9%) in the Rd Continuous, Rd18, and
MPT Arms, respectively.
Stroke (CVA) was reported as a serious (2.3%) or severe (2.0%) adverse drug reaction in the REVLIMID/dexamethasone group compared to 0.9% and 0.9%
respectively in the placebo/dexamethasone group. Discontinuation due to stroke (CVA) was 1.4% in REVLIMID/ dexamethasone group and 0.3% in the
placebo/dexamethasone group. In the NDMM study, CVA was reported as an adverse reaction (all grades: 0.8%, 0.6%, and 0.6%), as a serious adverse reaction (0.8%,
0.6 %, and 0.6%), or as a severe adverse reaction (0.6%, 0.6%, 0.2%) in the Rd Continuous, Rd18, and MPT arms respectively.
Other Adverse Reactions: After At Least One Prior Therapy for MM
In these 2 studies, the following adverse drug reactions (ADRs) not described above that occurred at ≥1% rate and of at least twice of the placebo percentage rate were
reported:
Blood and lymphatic system disorders: pancytopenia, autoimmune hemolytic anemia
Cardiac disorders: bradycardia, myocardial infarction, angina pectoris
Endocrine disorders: hirsutism
Eye disorders: blindness, ocular hypertension
Gastrointestinal disorders: gastrointestinal hemorrhage, glossodynia
General disorders and administration site conditions: malaise
Investigations: liver function tests abnormal, alanine aminotransferase increased
Nervous system disorders: cerebral ischemia
Psychiatric disorders: mood swings, hallucination, loss of libido
Reproductive system and breast disorders: erectile dysfunction
Respiratory, thoracic and mediastinal disorders: cough, hoarseness
Skin and subcutaneous tissue disorders: exanthem, skin hyperpigmentation
Myelodysplastic Syndromes:
A total of 148 patients received at least 1 dose of 10 mg REVLIMID in the del 5q MDS clinical study. At least one adverse reaction was reported in all of the 148
patients who were treated with the 10 mg starting dose of REVLIMID. The most frequently reported adverse reactions were related to blood and lymphatic system
disorders, skin and subcutaneous tissue disorders, gastrointestinal disorders, and general disorders and administrative site conditions.
Thrombocytopenia (61.5%; 91/148) and neutropenia (58.8%; 87/148) were the most frequently reported adverse reactions. The next most common adverse reactions
observed were diarrhea (48.6%; 72/148), pruritus (41.9%; 62/148), rash (35.8%; 53/148) and fatigue (31.1%; 46/148). Table 9 summarizes the adverse reactions that
were reported in ≥ 5% of the REVLIMID treated patients in the del 5q MDS clinical study. Table 10 summarizes the most frequently observed Grade 3 and Grade 4
adverse reactions regardless of relationship to treatment with REVLIMID. In the single-arm studies conducted, it is often not possible to distinguish adverse reactions
that are drug-related and those that reflect the patient’s underlying disease.
Table 9: Summary of Adverse Reactions Reported in ≥5% of the
REVLIMID Treated Patients in del 5q MDS Clinical Study
Body System
Adverse Reaction
a
10 mg Overall
(N=148)
Patients with at least one adverse reaction 148 (100)
Blood and Lymphatic System Disorders
Thrombocytopenia
Neutropenia
Anemia
Leukopenia
Febrile Neutropenia
91 (61)
87 (59)
17 (11)
12 (8)
8 (5)
Skin and Subcutaneous Tissue Disorders
Pruritus
Rash
Dry Skin
Contusion
Night Sweats
Sweating Increased
Ecchymosis
62 (42)
53 (36)
21 (14)
12 (8)
12 (8)
10 (7)
8 (5)
Reference ID: 4439576
19
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Body System
Adverse Reaction
a
10 mg Overall
(N=148)
Erythema 8 (5)
Gastrointestinal Disorders
Diarrhea
Constipation
Nausea
Abdominal Pain
Vomiting
Abdominal Pain Upper
Dry Mouth
Loose Stools
72 (49)
35 (24)
35 (24)
18 (12)
15 (10)
12 (8)
10 (7)
9 (6)
Respiratory, Thoracic and Mediastinal Disorders
Nasopharyngitis
Cough
Dyspnea
Pharyngitis
Epistaxis
Dyspnea Exertional
Rhinitis
Bronchitis
34 (23)
29 (20)
25 (17)
23 (16)
22 (15)
10 (7)
10 (7)
9 (6)
General Disorders and Administration Site Conditions
Fatigue
Pyrexia
Edema Peripheral
Asthenia
Edema
Pain
Rigors
Chest Pain
46 (31)
31 (21)
30 (20)
22 (15)
15 (10)
10 (7)
9 (6)
8 (5)
Musculoskeletal and Connective Tissue Disorders
Arthralgia
Back Pain
Muscle Cramp
Pain in Limb
Myalgia
Peripheral Swelling
32 (22)
31 (21)
27 (18)
16 (11)
13 (9)
12 (8)
Nervous System Disorders
Dizziness
Headache
Hypoesthesia
Dysgeusia
Peripheral Neuropathy
29 (20)
29 (20)
10 (7)
9 (6)
8 (5)
Infections and Infestations
Upper Respiratory Tract Infection
Pneumonia
Urinary Tract Infection
Sinusitis
Cellulitis
22 (15)
17 (11)
16 (11)
12 (8)
8 (5)
Metabolism and Nutrition Disorders
Hypokalemia
Anorexia
Hypomagnesemia
16 (11)
15 (10)
9 (6)
Investigations
Alanine Aminotransferase Increased 12 (8)
Psychiatric Disorders
Insomnia
Depression
15 (10)
8 (5)
Renal and Urinary Disorders
Dysuria 10 (7)
Vascular Disorders
Hypertension 9 ( 6)
Endocrine Disorders
Acquired Hypothyroidism 10 (7)
Cardiac Disorders
Palpitations 8 (5)
Reference ID: 4439576
20
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
a
Body System and adverse reactions are coded using the MedDRA dictionary. Body System
and adverse reactions are listed in descending order of frequency for the Overall column. A patient with multiple
occurrences of an adverse reaction is counted only once under the applicable Body System/Adverse Reaction.
Table 10: Most Frequently Observed Grade 3 and 4 Adverse Reactions
1
Regardless of Relationship to Study Drug Treatment in the del 5q MDS Clinical Study
Adverse Reactions
2
10 mg
(N=148)
Patients with at least one Grade 3/4 AE 131 (89)
Neutropenia 79 (53)
Thrombocytopenia 74 (50)
Pneumonia 11 (7)
Rash 10 (7)
Anemia 9 (6)
Leukopenia 8 (5)
Fatigue 7 (5)
Dyspnea 7 (5)
Back Pain 7 (5)
Febrile Neutropenia 6 (4)
Nausea 6 (4)
Diarrhea 5 (3)
Pyrexia 5 (3)
Sepsis 4 (3)
Dizziness 4 (3)
Granulocytopenia 3 (2)
Chest Pain 3 (2)
Pulmonary Embolism 3 (2)
Respiratory Distress 3 (2)
Pruritus 3 (2)
Pancytopenia 3 (2)
Muscle Cramp 3 (2)
Respirato
r
y Tract Infection 2 (1)
Upper Respiratory Tract Infection 2 (1)
Asthenia 2 (1)
Multi-organ Failure 2 (1)
Epistaxis 2 (1)
Hypoxia 2 (1)
Pleural Effusion 2 (1)
Pneumonitis 2 (1)
Pulmonary Hypertension 2 (1)
Vomiting 2 (1)
Sweating Increased 2 (1)
Arthralgia 2 (1)
Pain in Limb 2 (1)
Headache 2 (1)
Syncope 2 (1)
1
Adverse reactions with frequency ≥1% in the 10 mg Overall group. Grade 3 and 4 are based on National Cancer Institute Common
Toxicity Criteria version 2.
2
Adverse reactions are coded using the MedDRA dictionary. A patient with multiple occurrences of an adverse reaction is counted only
once in the adverse reaction category.
In other clinical studies of REVLIMID in MDS patients, the following serious adverse reactions (regardless of relationship to study drug treatment) not described in
Table 9 or 10 were reported:
Blood and lymphatic system disorders: warm type hemolytic anemia, splenic infarction, bone marrow depression, coagulopathy, hemolysis, hemolytic anemia,
refractory anemia
Cardiac disorders: cardiac failure congestive, atrial fibrillation, angina pectoris, cardiac arrest, cardiac failure, cardio-respiratory arrest, cardiomyopathy, myocardial
infarction, myocardial ischemia, atrial fibrillation aggravated, bradycardia, cardiogenic shock, pulmonary edema, supraventricular arrhythmia, tachyarrhythmia,
ventricular dysfunction
Ear and labyrinth disorders: vertigo
Endocrine disorders: Basedow’s disease
Gastrointestinal disorders: gastrointestinal hemorrhage, colitis ischemic, intestinal perforation, rectal hemorrhage, colonic polyp, diverticulitis, dysphagia, gastritis,
gastroenteritis, gastroesophageal reflux disease, obstructive inguinal hernia, irritable bowel syndrome, melena, pancreatitis due to biliary obstruction, pancreatitis,
perirectal abscess, small intestinal obstruction, upper gastrointestinal hemorrhage
General disorders and administration site conditions: disease progression, fall, gait abnormal, intermittent pyrexia, nodule, rigors, sudden death
Hepatobiliary disorders: hyperbilirubinemia, cholecystitis, acute cholecystitis, hepatic failure
Reference ID: 4439576
21
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
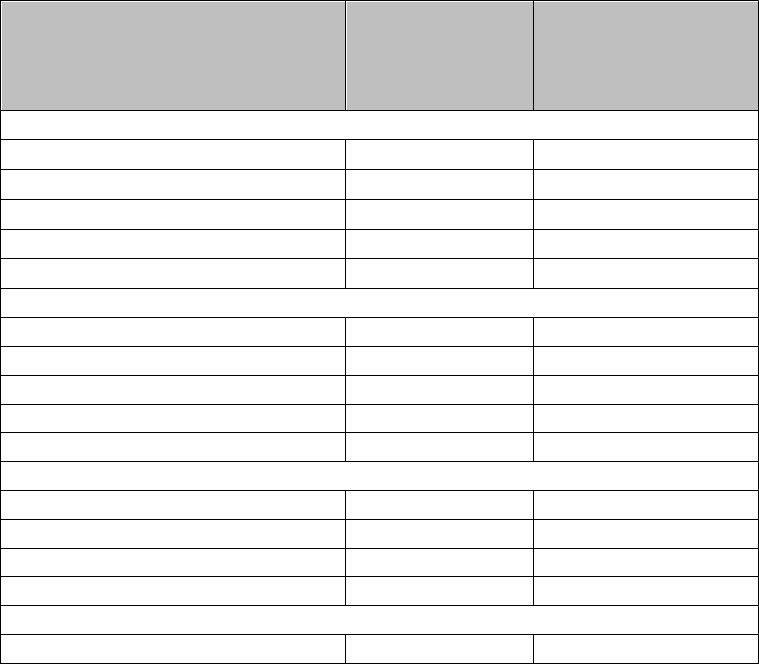
Immune system disorders: hypersensitivity
Infections and infestations: infection bacteremia, central line infection, clostridial infection, ear infection, Enterobacter sepsis, fungal infection, herpes viral infection
NOS, influenza, kidney infection, Klebsiella sepsis, lobar pneumonia, localized infection, oral infection, Pseudomonas infection, septic shock, sinusitis acute, sinusitis,
Staphylococcal infection, urosepsis
Injury, poisoning and procedural complications: femur fracture, transfusion reaction, cervical vertebral fracture, femoral neck fracture, fractured pelvis, hip fracture,
overdose, post procedural hemorrhage, rib fracture, road traffic accident, spinal compression fracture
Investigations: blood creatinine increased, hemoglobin decreased, liver function tests abnormal, troponin I increased
Metabolism and nutrition disorders: dehydration, gout, hypernatremia, hypoglycemia
Musculoskeletal and connective tissue disorders: arthritis, arthritis aggravated, gouty arthritis, neck pain, chondrocalcinosis pyrophosphate
Neoplasms benign, malignant and unspecified: acute leukemia, acute myeloid leukemia, bronchoalveolar carcinoma, lung cancer metastatic, lymphoma, prostate
cancer metastatic
Nervous system disorders: cerebrovascular accident, aphasia, cerebellar infarction, cerebral infarction, depressed level of consciousness, dysarthria, migraine, spinal
cord compression, subarachnoid hemorrhage, transient ischemic attack
Psychiatric disorders: confusional state
Renal and urinary disorders: renal failure, hematuria, renal failure acute, azotemia, calculus ureteric, renal mass
Reproductive system and breast disorders: pelvic pain
Respiratory, thoracic and mediastinal disorders: bronchitis, chronic obstructive airways disease exacerbated, respiratory failure, dyspnea exacerbated, interstitial
lung disease, lung infiltration, wheezing
Skin and subcutaneous tissue disorders: acute febrile neutrophilic dermatosis
Vascular system disorders: deep vein thrombosis, hypotension, aortic disorder, ischemia, thrombophlebitis superficial, thrombosis
Mantle Cell Lymphoma:
In the MCL trial, a total of 134 patients received at least 1 dose of REVLIMID. Their median age was 67 (range 43-83) years, 128/134 (96%) were Caucasian, 108/134
(81%) were males and 82/134 (61%) had duration of MCL for at least 3 years.
Table 11 summarizes the most frequently observed adverse reactions regardless of relationship to treatment with REVLIMID. Across the 134 patients treated in this
study, median duration of treatment was 95 days (1-1002 days). Seventy-eight patients (58%) received 3 or more cycles of therapy, 53 patients (40%) received 6 or
more cycles, and 26 patients (19%) received 12 or more cycles. Seventy-six patients (57%) underwent at least one dose interruption due to adverse reactions, and 51
patients (38%) underwent at least one dose reduction due to adverse reactions. Twenty-six patients (19%) discontinued treatment due to adverse reactions.
Table 11: Incidence of Adverse Reactions (≥10%) or Grade 3 / 4 AE (in at least 2 patients)
in Mantle Cell Lymphoma
Body System
Adverse Reaction
All Adverse Reactions
1
(N=134)
n (%)
Grade 3/4 Adverse
Reactions
2
(N=134)
n (%)
General disorders and administration site conditions
Fatigue 45 (34) 9 (7)
Pyrexia
$
31 (23) 3 (2)
Edema peripheral 21 (16) 0
Asthenia
$
19 (14) 4 (3)
General physical health deterioration 3 (2) 2 (1)
Gastrointestinal disorders
Diarrhea
$
42 (31) 8 (6)
Nausea
$
40 (30) 1 (<1)
Constipation 21 (16) 1 (<1)
Vomiting
$
16 (12) 1 (<1)
Abdominal pain
$
13 (10) 5 ( 4)
Musculoskeletal and connective tissue disorders
Back pain 18 (13) 2 (1)
Muscle spasms 17 (13) 1 (<1)
Arthralgia 11 (8) 2 (1)
Muscular weakness
$
8 (6) 2 ( 1)
Respiratory, thoracic and mediastinal disorders
Cough 38 (28) 1 (<1)
Reference ID: 4439576
22
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
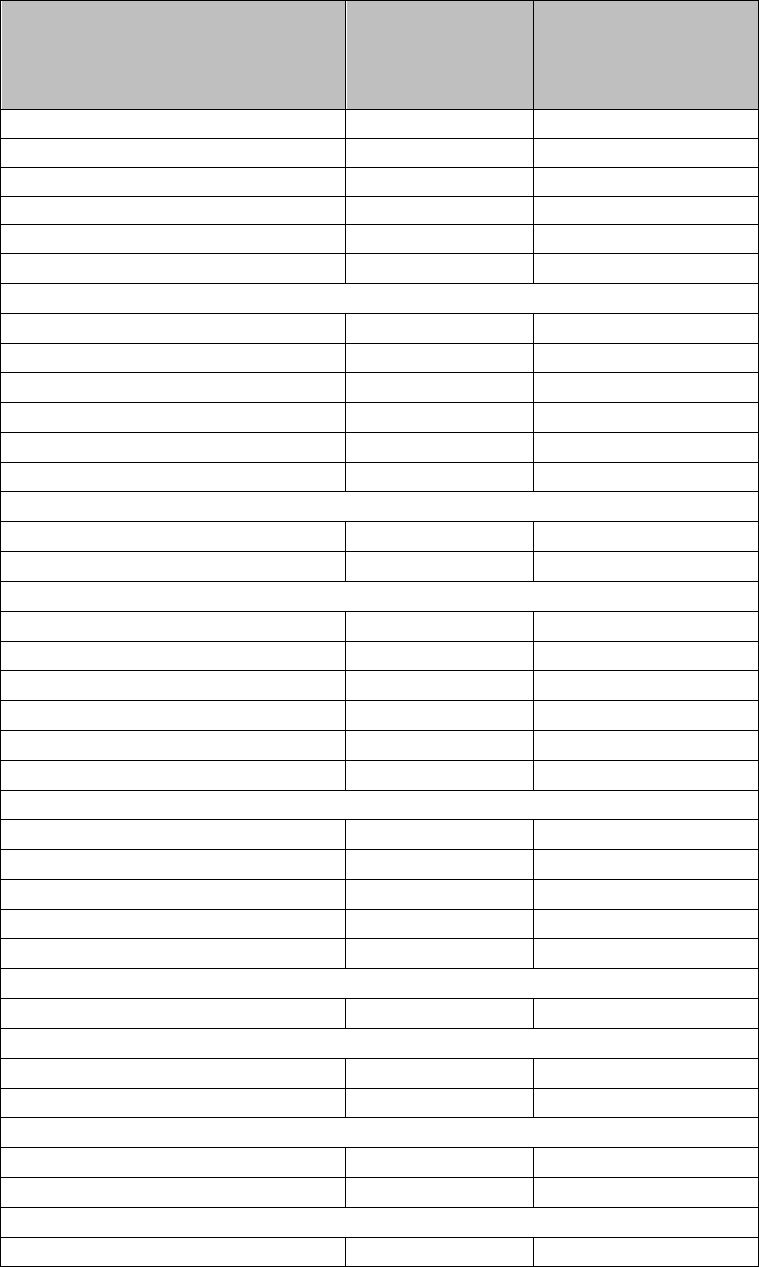
Body System
Adverse Reaction
All Adverse Reactions
1
(N=134)
n (%)
Grade 3/4 Adverse
Reactions
2
(N=134)
n (%)
Dyspnea
$
24 (18) 8 (6)
Pleural Effusion 10 (7) 2 (1)
Hypoxia 3 (2) 2 (1)
Pulmonary embolism 3 (2) 2 ( 1)
Respiratory distress
$
2 (1) 2 (1)
Oropharyngeal pain 13 (10) 0
Infections and infestations
Pneumonia
@ $
19 (14) 12 (9)
Upper respiratory tract infection 17 (13) 0
Cellulitis
$
3 (2) 2 (1)
Bacteremia
$
2 (1) 2 (1)
Staphylococcal sepsis
$
2 (1) 2 (1)
Urinary tract infection
$
5 (4) 2 (1)
Skin and subcutaneous tissue disorders
Rash
+
30 (22) 2 (1)
Pruritus 23 (17) 1 (<1)
Blood and lymphatic system disorders
Neutropenia 65 (49) 58 (43)
Thrombocytopenia
% $
48 (36) 37 (28)
Anemia
$
41 (31) 15 (11)
Leukopenia
$
20 (15) 9 (7)
Lymphopenia 10 ( 7) 5 (4)
Febrile neutropenia
$
8 (6) 8 (6)
Metabolism and nutrition disorders
Decreased appetite 19 (14) 1 (<1)
Hypokalemia 17 (13) 3 (2)
Dehydration
$
10 (7) 4 (3)
Hypocalcemia 4 (3) 2 (1)
Hyponatremia 3 (2) 3 (2)
Renal and urinary disorders
Renal failure
$
5 (4) 2 (1)
Vascular disorders
Hypotension
@ $
9 (7) 4 (3)
Deep vein thrombosis
$
5 (4) 5 (4)
Neoplasms benign, malignant and unspecified (including cysts and polyps)
Tumor flare 13 (10) 0
Squamous cell carcinoma of skin
$
4 (3) 4 (3)
Investigations
Weight decreased 17 (13) 0
1
-MCL trial AEs – All treatment emergent AEs with ≥10% of subjects.
2
-MCL trial Grade 3/4 AEs – All treatment-emergent Grade 3/4 AEs in 2 or more subjects.
$
-MCL trial Serious AEs – All treatment-emergent SAEs in 2 or more subjects.
@
- Adverse reactions where at least one resulted in a fatal outcome.
%
- Adverse reactions where at least one was considered to be Life Threatening (if the outcome of the event was death, it is included with death cases).
#
- All adverse reactions under Body System of Infections except for rare infections of Public Health interest will be considered listed.
+
- All adverse reactions under HLT of Rash will be considered listed.
Reference ID: 4439576
23
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
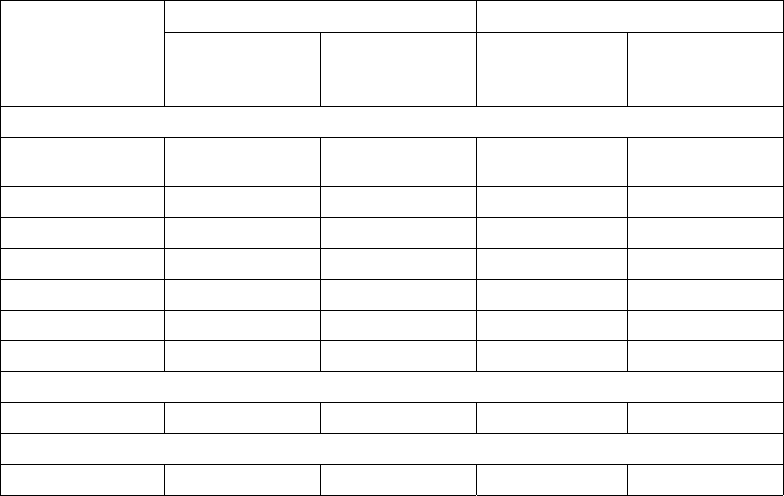
The following adverse reactions which have occurred in other indications including another MCL study and not described above have been reported (1%-10%) in
patients treated with REVLIMID monotherapy for mantle cell lymphoma.
Cardiac disorder: Cardiac failure
Ear and labyrinth disorders: Vertigo
General disorders and administration site conditions: Chills
Infections and infestations: Respiratory tract infection, sinusitis, nasopharyngitis, oral herpes
Musculoskeletal and connective tissue disorders: Pain in extremity
Nervous system disorders: Dysgeusia, headache, neuropathy peripheral, lethargy
Psychiatric disorders: Insomnia
Skin and subcutaneous tissue disorders: Dry skin, night sweats
The following serious adverse reactions not described above and reported in 2 or more patients treated with REVLIMID monotherapy for mantle cell lymphoma.
Blood and lymphatic system disorders: Neutropenia
Cardiac disorder: Myocardial infarction (including acute MI), supraventricular tachycardia
Infections and infestations: Clostridium difficile colitis, sepsis
Neoplasms benign, malignant and unspecified (including cysts and polyps): Basal cell carcinoma
Respiratory, thoracic, and mediastinal disorders: Chronic obstructive pulmonary disease, pulmonary embolism
Follicular Lymphoma or Marginal Zone Lymphoma
The safety of REVLIMID/ rituximab was evaluated in 398 patients with either previously treated follicular lymphoma or marginal zone lymphoma in two clinical trials;
AUGMENT (N=176) and MAGNIFY (N=222) [see Clinical Studies (14.4)]. Subjects were 18 years or older in age, had an ECOG PS ≤2, ANC ≥1,000 cells/mm
3
and
platelets≥ 75,000/mm
3
(unless secondary to bone marrow involvement by lymphoma), hemoglobin ≥8g/dL, AST and ALT ≤ 3x ULN (unless documented liver
involvement with lymphoma, and creatinine clearance of ≥ 30mL/min. Subjects with active HIV, hepatitis B or C were not eligible.
In the AUGMENT trial, patients received REVLIMID 20 mg daily by mouth on days 1 – 21 of each 28 day cycle with rituximab 375 mg/m
2
weekly (days 1, 8, 15 and
22 in cycle 1) then on day 1 of cycles 2-5 (n=176) or placebo with rituximab 375 mg/m
2
weekly (days 1, 8, 15 and 22 in cycle 1) then on day 1 of cycles 2-5 (n=180) for
up to 12 cycles. In the MAGNIFY trial, patients received REVLIMID 20 mg by mouth daily, days 1-21 of each 28 day cycle with rituximab 375 mg/m
2
weekly (days 1,
8, 15 and 22 in cycle 1) then on day 1 of cycles 3, 7, 9 and 11 in the induction phase of the trial (n=222). In the AUGMENT trial, 88.1% of patients completed at least 6
cycles of REVLIMID/rituximab, and 71% of patients completed 12 cycles. In the ongoing MAGNIFY trial as of May 1, 2017, 62.2% of patients completed at least 6
cycles of REVLIMID/rituximab, and 30.6% of patients completed 12 cycles.
Across both clinical trials (AUGMENT and MAGNIFY), patients had a median age of 64.5 years (26 to 91); 49% were male; and 81% were White.
Fatal adverse reactions occurred in 6 patients (1.5%) receiving REVLIMID/rituximab. Fatal adverse reactions (1 each) included cardio-respiratory arrest, arrhythmia,
cardiopulmonary failure, multiple organ dysfunction syndrome, sepsis, and acute kidney injury. Serious adverse reactions occurred in 26% of patients receiving
REVLIMID/rituximab on AUGMENT and 29% on MAGNIFY. The most frequent serious adverse reaction that occurred in ≥ 2.5% of patients in the
REVLIMID/rituximab arm was febrile neutropenia (3%). Permanent discontinuation of REVLIMID or rituximab due to an adverse reaction occurred in 14.6% of
patients in the REVLIMID/rituximab arm. The most common adverse reaction (in at least 1%) requiring permanent discontinuation of REVLIMID or rituximab was
neutropenia (4.8%).
The most common adverse reactions occurring in at least 20% of subjects were; neutropenia (48%), fatigue (37%), diarrhea (32%), constipation (27%), nausea (21%),
and cough (20%).
Table 12: All Grade Adverse Reactions ( ≥5%) or Grade 3/4 Adverse Reactions ( ≥1%) in Patients with FL and
MZL with a Difference Between Arms of >1% When Compared to Control Arm in AUGMENT Trial
Body System
Adverse Reaction*
All Adverse Reactions
1
Grade 3 / 4 Adverse Reactions
2
REVLIMID +
Rituximab Arm
(N=176)
n (%)
Rituximab + Placebo
(Control Arm)
(N=180)
n (%)
REVLIMID +
Rituximab Arm
(N=176)
n (%)
Rituximab + Placebo
(Control Arm)
(N=180)
n (%)
Infections and infestations
Upper respiratory tract
infection
32 (18) 23 (13) 2 (1.1) 4 (2.2)
Influenza
%
17 (10) 8 (4.4) 1 (< 1) 0 (0)
Pneumonia
3,$,%
13 (7) 6 (3.3) 6 (3.4) 4 (2.2)
Sinusitis 13 (7) 5 (2.8) 0 (0) 0 (0)
Urinary tract infection
$
13 (7) 7 (3.9) 1 (< 1) 1 (< 1)
Bronchitis 8 (4.5) 6 (3.3) 2 (1.1) 0 (0)
Gastroenteritis
$
6 (3.4) 4 (2.2) 2 (1.1) 0 (0)
Neoplasms benign, malignant and unspecified (including cysts and polyps)
Tumor flare
$
19 (11) 1 (< 1) 1 (< 1) 0 (0)
Blood and lymphatic disorders
Neutropenia
3,$, %
102 (58) 40 (22) 88 (50) 23 (13)
Reference ID: 4439576
24
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Body System
Adverse Reaction*
All Adverse Reactions
1
Grade 3 / 4 Adverse Reactions
2
REVLIMID +
Rituximab Arm
(N=176)
n (%)
Rituximab + Placebo
(Control Arm)
(N=180)
n (%)
REVLIMID +
Rituximab Arm
(N=176)
n (%)
Rituximab + Placebo
(Control Arm)
(N=180)
n (%)
Leukopenia
$,%
36 (20) 17 (9) 12 (7) 3 (1.7)
Anemia
3,$
28 (16) 8 (4.4) 8 (4.5) 1 (< 1)
Thrombocytopenia
3,$,%
26 (15) 8 (4.4) 4 (2.3) 2 (1.1)
Lymphopenia 8 (4.5) 14 (8) 5 (2.8) 2 (1.1)
Febrile
Neutropenia
3,$,%
5 (2.8) 1 (< 1) 5 (2.8) 1 (< 1)
Metabolism and nutrition disorders
Decreased Appetite 23 (13) 11 (6) 2 (1.1) 0 (0)
Hypokalemia
%
14 (8) 5 (2.8) 4 (2.3) 0 (0)
Hyperuricemia 10 (6) 8 (4.4) 1 (< 1) 1 (< 1)
Nervous system disorders
Headache 26 (15) 17 (9) 1 (< 1) 0 (0)
Dizziness 15 (9) 9 (5) 0 (0) 0 (0)
Vascular disorders
Hypotension
%
9 (5) 1 (< 1) 1 (< 1) 0 (0)
Thromboembolic
events
a,$
8 (4.5) 2 (1.1) 4 (2.3) 2 (1.1)
Respiratory, thoracic and mediastinal disorders
Cough
b
43 (24) 35 (19) 1 (< 1) 0 (0)
Dyspnea
$
19 (11) 8 (4.4) 2 (1.1) 1 (< 1)
Oropharyngeal pain 10 (6) 8 (4.4) 0 (0) 0 (0)
Pulmonary
Embolism
3,$
4 (2.3) 1 (< 1) 4 (2.3) 1 (< 1)
Chronic obstructive
pulmonary disease
$
3 (1.7) 0 (0) 2 (1.1) 0 (0)
Respiratory failure
3,$
2 (1.1) 1 (< 1) 2 (1.1) 0 (0)
Gastrointestinal disorders
Diarrhea
$,%
55 (31) 41 (23) 5 (2.8) 0 (0)
Constipation 46 (26) 25 (14) 0 (0) 0 (0)
Abdominal pain
c ,$
32 (18) 20 (11) 2 (1.1) 0 (0)
Vomiting
$
17 (10) 13 (7) 0 (0) 0 (0)
Dyspepsia 16 (9) 5 (2.8) 0 (0) 0 (0)
Stomatitis 9 (5) 7 (3.9) 0 (0) 0 (0)
Skin and subcutaneous tissue disorders
Rash
$,d
39 (22) 14 (8) 5 (2.8) 2 (1.1)
Pruritus
$,e
36 (20) 9 (5) 2 (1.1) 0 (0)
Dry skin 9 (5) 6 (3.3) 0 (0) 0 (0)
Dermatitis acneiform 8 (4.5) 0 (0) 2 (1.1) 0 (0)
Musculoskeletal and connective tissue disorders
Muscle Spasms 23 (13) 9 (5) 1 (< 1) 1 (< 1)
Pain in Extremity
$
8 (4.5) 9 (5) 2 (1) 0 (0)
Renal disorders
Acute Kidney
Injury
3,$,@,%
3 (1.7) 0 (0) 2 (1.1) 0 (0)
Reference ID: 4439576
25
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Body System
Adverse Reaction*
All Adverse Reactions
1
Grade 3 / 4 Adverse Reactions
2
REVLIMID +
Rituximab Arm
(N=176)
n (%)
Rituximab + Placebo
(Control Arm)
(N=180)
n (%)
REVLIMID +
Rituximab Arm
(N=176)
n (%)
Rituximab + Placebo
(Control Arm)
(N=180)
n (%)
Cardiac disorders
Supraventricular
tachycardia
3,$
2 (1.1) 0 (0) 2 (1.1) 0 (0)
General disorders and administration site conditions
Fatigue 38 (22) 33 (18) 2 (1.1) 1 (< 1)
Pyrexia
3,$
37 (21) 27 (15) 1 (< 1) 3 (1.7)
Asthenia
$,%
24 (14) 19 (11) 2 (1.1) 1 (< 1)
Edema Peripheral
$
23 (13) 16 (9) 0 (0) 0 (0)
Chills 14 (8) 8 (4.4) 0 (0) 0 (0)
Malaise 13 (7) 10 (6) 0 (0) 0 (0)
Influenza like illness 9 (5) 7 (3.9) 0 (0) 0 (0)
Psychiatric disorders
Insomnia 14 (8) 11 (6) 0 (0) 0 (0)
Investigations
Alanine
Aminotransferase
Increased
18 (10) 15 (8) 3 (1.7) 1 (< 1)
WBC count decreased 16 (9) 13 (7) 5 (2.8) 2 (1.1)
Lymphocyte count
decreased
12 (7) 12 (7) 6 (3.4) 2 (1.1)
Blood bilirubin
increased
10 (6) 0 (0) 0 (0) 0 (0)
Weight Decreased 12 (7) 2 (1.1) 0 (0) 0 (0)
Note: Adverse reactions are coded to body system/adverse reaction using MedDRA 21. A patient with multiple occurrences of an adverse
reaction is counted only once under the applicable Body System/Adverse reaction.
1
All treatment-emergent AEs in at least 5% of patients in the REVLIMID + rituximab group and at least 1% higher frequency (%) than the
rituximab + placebo group (control arm).
2
All grade 3 or 4 treatment-emergent AEs in at least 1% of patients in the REVLIMID + rituximab group and at least 1% higher frequency (%)
than the rituximab + placebo group (control arm).
3
All serious treatment-emergent AEs in at least 1% of patients in the REVLIMID + rituximab group and at least 1% higher frequency (%) than
the rituximab + placebo group (control arm).
$
Serious ADR reported.
@
- adverse reactions in which at least one resulted in a fatal outcome.
%
- adverse reactions in which at least one was considered to be life threatening (if the outcome of the reaction was death, it is included with death
cases).
*Adverse Reactions for combined ADR terms (based on relevant TEAE PTs [per MedDRA version 21.0]):
a “Thromboembolic events” combined term includes the following PTs: pulmonary embolism, deep vein thrombosis, cerebrovascular accident,
embolism, and thrombosis.
b “Cough” combined AE term includes the following PTs: cough and productive cough.
c “Abdominal pain” combined AE term includes the following PTs: abdominal pain and abdominal pain upper.
d “Rash” combined AE term includes the following PTs: rash maculo-papular, rash erythematous, rash macular, rash papular, rash pruritic, and
rash generalized.
e “Pruritus” combined AE term includes the following PTs: pruritius, pruritus generalized, rash pruritic, and pruritus allergic.
Postmarketing Experience
The following adverse drug reactions have been identified from the worldwide post-marketing experience with REVLIMID. Because these reactions are reported
voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure [see
Warnings and Precautions Section (5.8 to 5.11, and 5.13)]
Endocrine disorders: Hypothyroidism, hyperthyroidism
Hepatobiliary disorders: Hepatic failure (including fatality), toxic hepatitis, cytolytic hepatitis, cholestatic hepatitis, mixed cytolytic/cholestatic hepatitis, transient
abnormal liver laboratory tests
Immune system disorders: Angioedema, acute graft-versus-host disease (following allogeneic hematopoietic transplant), solid organ transplant rejection
Infections and infestations: Viral reactivation (such as hepatitis B virus and herpes zoster), progressive multifocal leukoencephalopathy (PML)
Neoplasms benign, malignant and unspecified (including cysts and polyps): Tumor lysis syndrome, tumor flare reaction
Respiratory, thoracic and mediastinal disorders: Pneumonitis
Skin and subcutaneous tissue disorders: Stevens-Johnson Syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms (DRESS)
Reference ID: 4439576
26
6.2
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

7 DRUG INTERACTIONS
7.1 Digoxin
When digoxin was co-administered with multiple doses of REVLIMID (10 mg/day) the digoxin C
max
and AUC
inf
were increased by 14%. Periodically monitor digoxin
plasma levels, in accordance with clinical judgment and based on standard clinical practice in patients receiving this medication, during administration of REVLIMID.
7.2 Concomitant Therapies That May Increase the Risk of Thrombosis
Erythropoietic agents, or other agents that may increase the risk of thrombosis, such as estrogen containing therapies, should be used with caution after making a
benefit-risk assessment in patients receiving REVLIMID [see Warnings and Precautions (5.4)].
7.3 Warfarin
Co-administration of multiple doses of REVLIMID (10 mg/day) with a single dose of warfarin (25 mg) had no effect on the pharmacokinetics of lenalidomide or R- and
S-warfarin. Expected changes in laboratory assessments of PT and INR were observed after warfarin administration, but these changes were not affected by
concomitant REVLIMID administration. It is not known whether there is an interaction between dexamethasone and warfarin. Close monitoring of PT and INR is
recommended in patients with MM taking concomitant warfarin.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in females exposed to REVLIMID during pregnancy as well as female partners of male
patients who are exposed to REVLIMID. This registry is also used to understand the root cause for the pregnancy. Report any suspected fetal exposure to REVLIMID
to the FDA via the MedWatch program at 1-800-FDA-1088 and also to Celgene Corporation at 1-888-423-5436.
Risk Summary
Based on the mechanism of action [see Clinical Pharmacology (12.1)] and findings from animal studies [see Data], REVLIMID can cause embryo-fetal harm when
administered to a pregnant female and is contraindicated during pregnancy [see Boxed Warning, Contraindications (4.1), and Use in Specific Populations (5.1)].
REVLIMID is a thalidomide analogue. Thalidomide is a human teratogen, inducing a high frequency of severe and life-threatening birth defects such as amelia
(absence of limbs), phocomelia (short limbs), hypoplasticity of the bones, absence of bones, external ear abnormalities (including anotia, micropinna, small or absent
external auditory canals), facial palsy, eye abnormalities (anophthalmos, microphthalmos), and congenital heart defects. Alimentary tract, urinary tract, and genital
malformations have also been documented and mortality at or shortly after birth has been reported in about 40% of infants.
Lenalidomide caused thalidomide-type limb defects in monkey offspring. Lenalidomide crossed the placenta after administration to pregnant rabbits and pregnant rats
[see Data]. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential risk to a
fetus.
If pregnancy does occur during treatment, immediately discontinue the drug. Under these conditions, refer patient to an obstetrician/gynecologist experienced in
reproductive toxicity for further evaluation and counseling. Report any suspected fetal exposure to REVLIMID to the FDA via the MedWatch program at 1-800-FDA-
1088 and also to Celgene Corporation at 1-888-423-5436.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect,
loss, or other adverse outcomes. The estimated background risk in the U.S. general population of major birth defects is 2%-4% and of miscarriage is 15%-20% of
clinically recognized pregnancies.
Data
Animal data
In an embryo-fetal developmental toxicity study in monkeys, teratogenicity, including thalidomide-like limb defects, occurred in offspring when pregnant monkeys
received oral lenalidomide during organogenesis. Exposure (AUC) in monkeys at the lowest dose was 0.17 times the human exposure at the maximum recommended
human dose (MRHD) of 25 mg. Similar studies in pregnant rabbits and rats at 20 times and 200 times the MRHD respectively, produced embryo lethality in rabbits and
no adverse reproductive effects in rats.
In a pre- and post-natal development study in rats, animals received lenalidomide from organogenesis through lactation. The study revealed a few adverse effects on
the offspring of female rats treated with lenalidomide at doses up to 500 mg/kg (approximately 200 times the human dose of 25 mg based on body surface area). The
male offspring exhibited slightly delayed sexual maturation and the female offspring had slightly lower body weight gains during gestation when bred to male offspring.
As with thalidomide, the rat model may not adequately address the full spectrum of potential human embryo-fetal developmental effects for lenalidomide.
Following daily oral administration of lenalidomide from Gestation Day 7 through Gestation Day 20 in pregnant rabbits, fetal plasma lenalidomide concentrations were
approximately 20-40% of the maternal C
max.
Following a single oral dose to pregnant rats, lenalidomide was detected in fetal plasma and tissues; concentrations of
radioactivity in fetal tissues were generally lower than those in maternal tissues. These data indicated that lenalidomide crossed the placenta.
8.2 Lactation
Risk Summary
There is no information regarding the presence of lenalidomide in human milk, the effects of REVLIMID on the breastfed child, or the effects of REVLIMID on milk
production. Because many drugs are excreted in human milk and because of the potential for adverse reactions in breastfed children from REVLIMID, advise women
not to breastfeed during treatment with REVLIMID.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Reference ID: 4439576
27
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

REVLIMID can cause fetal harm when administered during pregnancy [see Use in Specific Populations (8.1)]. Verify the pregnancy status of females of reproductive
potential prior to initiating REVLIMID therapy and during therapy. Advise females of reproductive potential that they must avoid pregnancy 4 weeks before therapy,
while taking REVLIMID, during dose interruptions and for at least 4 weeks after completing therapy.
Females of reproductive potential must have 2 negative pregnancy tests before initiating REVLIMID. The first test should be performed within 10-14 days, and the
second test within 24 hours prior to prescribing REVLIMID. Once treatment has started and during dose interruptions, pregnancy testing for females of reproductive
potential should occur weekly during the first 4 weeks of use, then pregnancy testing should be repeated every 4 weeks in females with regular menstrual cycles. If
menstrual cycles are irregular, the pregnancy testing should occur every 2 weeks. Pregnancy testing and counseling should be performed if a patient misses her period
or if there is any abnormality in her menstrual bleeding. REVLIMID treatment must be discontinued during this evaluation.
Contraception
Females
Females of reproductive potential must commit either to abstain continuously from heterosexual sexual intercourse or to use 2 methods of reliable birth control
simultaneously: one highly effective form of contraception – tubal ligation, IUD, hormonal (birth control pills, injections, hormonal patches, vaginal rings, or implants),
or partner’s vasectomy, and 1 additional effective contraceptive method – male latex or synthetic condom, diaphragm, or cervical cap. Contraception must begin 4
weeks prior to initiating treatment with REVLIMID, during therapy, during dose interruptions, and continuing for 4 weeks following discontinuation of REVLIMID
therapy. Reliable contraception is indicated even where there has been a history of infertility, unless due to hysterectomy. Females of reproductive potential should be
referred to a qualified provider of contraceptive methods, if needed.
Males
Lenalidomide is present in the semen of males who take REVLIMID. Therefore, males must always use a latex or synthetic condom during any sexual contact with
females of reproductive potential while taking REVLIMID and for up to 4 weeks after discontinuing REVLIMID, even if they have undergone a successful vasectomy.
Male patients taking REVLIMID must not donate sperm.
8.4 Pediatric Use
Safety and effectiveness have not been established in pediatric patients.
8.5 Geriatric Use
MM In Combination: Overall, of the 1613 patients in the NDMM study who received study treatment, 94% (1521 /1613) were 65 years of age or older, while 35%
(561/1613) were over 75 years of age. The percentage of patients over age 75 was similar between study arms (Rd Continuous: 33%; Rd18: 34%; MPT: 33%). Overall,
across all treatment arms, the frequency in most of the adverse reaction categories (eg, all adverse reactions, grade 3/4 adverse reactions, serious adverse reactions) was
higher in older (> 75 years of age) than in younger (≤ 75 years of age) subjects. Grade 3 or 4 adverse reactions in the General Disorders and Administration Site
Conditions body system were consistently reported at a higher frequency (with a difference of at least 5%) in older subjects than in younger subjects across all treatment
arms. Grade 3 or 4 adverse reactions in the Infections and Infestations, Cardiac Disorders (including cardiac failure and congestive cardiac failure), Skin and
Subcutaneous Tissue Disorders, and Renal and Urinary Disorders (including renal failure) body systems were also reported slightly, but consistently, more frequently
(<5% difference), in older subjects than in younger subjects across all treatment arms. For other body systems (e.g., Blood and Lymphatic System Disorders, Infections
and Infestations, Cardiac Disorders, Vascular Disorders), there was a less consistent trend for increased frequency of grade 3/4 adverse reactions in older vs younger
subjects across all treatment arms Serious adverse reactions were generally reported at a higher frequency in the older subjects than in the younger subjects across all
treatment arms.
MM Maintenance Therapy: Overall, 10% (106/1018) of patients were 65 years of age or older, while no patients were over 75 years of age. Grade 3 or 4 adverse
reactions were higher in the REVLIMID arm (more than 5% higher) in the patients 65 years of age or older versus younger patients. The frequency of Grade 3 or 4
adverse reactions in the Blood and Lymphatic System Disorders were higher in the REVLIMID arm (more than 5% higher) in the patients 65 years of age or older
versus younger patients. There were not a sufficient number of patients 65 years of age or older in REVLIMID maintenance studies who experienced either a serious
adverse reaction, or discontinued therapy due to an adverse reaction to determine whether elderly patients respond relative to safety differently from younger patients.
MM After At Least One Prior Therapy: Of the 703 MM patients who received study treatment in Studies 1 and 2, 45% were age 65 or over while 12% of patients were
age 75 and over. The percentage of patients age 65 or over was not significantly different between the REVLIMID/dexamethasone and placebo/dexamethasone groups.
Of the 353 patients who received REVLIMID/dexamethasone, 46% were age 65 and over. In both studies, patients > 65 years of age were more likely than patients ≤
65 years of age to experience DVT, pulmonary embolism, atrial fibrillation, and renal failure following use of REVLIMID. No differences in efficacy were observed
between patients over 65 years of age and younger patients.
Of the 148 patients with del 5q MDS enrolled in the major study, 38% were age 65 and over, while 33% were age 75 and over. Although the overall frequency of
adverse reactions (100%) was the same in patients over 65 years of age as in younger patients, the frequency of serious adverse reactions was higher in patients over 65
years of age than in younger patients (54% vs. 33%). A greater proportion of patients over 65 years of age discontinued from the clinical studies because of adverse
reactions than the proportion of younger patients (27% vs.16%). No differences in efficacy were observed between patients over 65 years of age and younger patients.
Of the 134 patients with MCL enrolled in the MCL trial, 63% were age 65 and over, while 22% of patients were age 75 and over. The overall frequency of adverse
reactions was similar in patients over 65 years of age and in younger patients (98% vs. 100%). The overall incidence of grade 3 and 4 adverse reactions was also similar
in these 2 patient groups (79% vs. 78%, respectively). The frequency of serious adverse reactions was higher in patients over 65 years of age than in younger patients
(55% vs. 41%). No differences in efficacy were observed between patients over 65 years of age and younger patients.
FL or MZL in Combination: Overall, 48% (282/590) of patients were 65 years of age or older, while 14% (82/590) of patients were over 75 years of age. The overall
frequency of adverse reactions was similar in patients 65 years of age or older and younger patients for both studies pooled (98%). Grade 3 or 4 adverse reactions were
higher in the REVLIMID arm (more than 5% higher) in the patients 65 years of age or older versus younger patients (71% versus 59%). The frequency of Grade 3 or 4
adverse reactions were higher in the REVLIMID arm (more than 5% higher) in the patients 65 years of age or older versus younger patients in the Blood and Lymphatic
System Disorders (47% versus 40%) and Infections and Infestations (16% versus 11%). Serious adverse reactions were higher in the REVLIMID arm (more than 5%
higher) in the patients 65 years of age or older versus younger patients (37% versus 18%). The frequency of serious adverse reactions were higher in the REVLIMID
arm (more than 5% higher) in the patients 65 years of age or older versus younger patients in Infections and Infestations (15% versus 6%).
Since elderly patients are more likely to have decreased renal function, care should be taken in dose selection. Monitor renal function.
8.6 Renal Impairment
Adjust the starting dose of REVLIMID based on the creatinine clearance value and for patients on dialysis [see Dosage and Administration (2.5)].
Reference ID: 4439576
28
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
12
10 OVERDOSAGE
There is no specific experience in the management of REVLIMID overdose in patients with MM, MDS, MCL, FL, or MZL. In dose-ranging studies in healthy subjects,
some were exposed to up to 200 mg (administered 100 mg BID) and in single-dose studies, some subjects were exposed to up to 400 mg. Pruritus, urticaria, rash, and
elevated liver transaminases were the primary reported AEs. In clinical trials, the dose-limiting toxicity was neutropenia and thrombocytopenia.
11 DESCRIPTION
REVLIMID, a thalidomide analogue, is an immunomodulatory agent with antiangiogenic and antineoplastic properties. The chemical name is 3-(4-amino-1-oxo 1,3-
dihydro-2H-isoindol-2-yl) piperidine-2,6-dione and it has the following chemical structure:
N
N
H
O
O
O
NH
2
3-(4-amino-1-oxo 1,3-dihydro-2H-isoindol-2-yl) piperidine-2,6-dione
The empirical formula for lenalidomide is C
13
H
13
N
3
O
3,
and the gram molecular weight is 259.3.
Lenalidomide is an off-white to pale-yellow solid powder. It is soluble in organic solvent/water mixtures, and buffered aqueous solvents. Lenalidomide is more soluble
in organic solvents and low pH solutions. Solubility was significantly lower in less acidic buffers, ranging from about 0.4 to 0.5 mg/ml. Lenalidomide has an
asymmetric carbon atom and can exist as the optically active forms S(-) and R(+), and is produced as a racemic mixture with a net optical rotation of zero.
REVLIMID is available in 2.5 mg, 5 mg, 10 mg, 15 mg, 20 mg and 25 mg capsules for oral administration. Each capsule contains lenalidomide as the active ingredient
and the following inactive ingredients: lactose anhydrous, microcrystalline cellulose, croscarmellose sodium, and magnesium stearate. The 5 mg and 25 mg capsule
shell contains gelatin, titanium dioxide and black ink. The 2.5 mg and 10 mg capsule shell contains gelatin, FD&C blue #2, yellow iron oxide, titanium dioxide and
black ink. The 15 mg capsule shell contains gelatin, FD&C blue #2, titanium dioxide and black ink. The 20 mg capsule shell contains gelatin, FD&C blue #2, yellow
iron oxide, titanium dioxide and black ink.
CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Lenalidomide is an analogue of thalidomide with immunomodulatory, antiangiogenic, and antineoplastic properties. Cellular activities of lenalidomide are mediated
through its target cereblon, a component of a cullin ring E3 ubiquitin ligase enzyme complex. In vitro, in the presence of drug, substrate proteins (including Aiolos,
Ikaros, and CK1α) are targeted for ubiquitination and subsequent degradation leading to direct cytotoxic and immunomodulatory effects. Lenalidomide inhibits
proliferation and induces apoptosis of certain hematopoietic tumor cells including MM, mantle cell lymphoma, and del (5q) myelodysplastic syndromes, follicular
lymphoma and marginal zone lymphoma in vitro. Lenalidomide causes a delay in tumor growth in some in vivo nonclinical hematopoietic tumor models including MM.
Immunomodulatory properties of lenalidomide include increased number and activation of T cells and natural killer (NK) cells leading to direct and enhanced antibody-
dependent cell-mediated cytotoxicity (ADCC) via increased secretion of interleukin-2 and interferon-gamma, increased numbers of NKT cells, and inhibition of pro-
inflammatory cytokines (e.g., TNF-α and IL-6) by monocytes. In MM cells, the combination of lenalidomide and dexamethasone synergizes the inhibition of cell
proliferation and the induction of apoptosis. The combination of lenalidomide and rituximab increases ADCC and direct tumor apoptosis in follicular lymphoma cells
and increases ADCC in marginal zone lymphoma cells compared to rituximab alone in vitro.
Reference ID: 4439576
29
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

12.2 Pharmacodynamics
Cardiac Electrophysiology
The effect of lenalidomide on the QTc interval was evaluated in 60 healthy male subjects in a thorough QT study. At a dose two times the maximum recommended
dose, lenalidomide did not prolong the QTc interval. The largest upper bound of the two-sided 90% CI for the mean differences between lenalidomide and placebo was
below 10 ms.
12.3 Pharmacokinetics
Absorption
Lenalidomide is rapidly absorbed following oral administration. Following single and multiple doses of REVLIMID in patients with MM or MDS, the maximum
plasma concentrations occurred between 0.5 and 6 hours post-dose. The single and multiple dose pharmacokinetic disposition of lenalidomide is linear with AUC and
C
max
values increasing proportionally with dose. Multiple doses of REVLIMID at the recommended dosage does not result in drug accumulation.
Administration of a single 25 mg dose of REVLIMID with a high-fat meal in healthy subjects reduces the extent of absorption, with an approximate 20% decrease in
AUC and 50% decrease in C
max
. In the trials where the efficacy and safety were established for REVLIMID, the drug was administered without regard to food intake.
REVLIMID can be administered with or without food.
The oral absorption rate of lenalidomide in patients with MCL is similar to that observed in patients with MM or MDS.
Distribution
In vitro [
14
C]-lenalidomide binding to plasma proteins is approximately 30%.
Lenalidomide is present in semen at 2 hours (1379 ng/ejaculate) and 24 hours (35 ng/ejaculate) after the administration of REVLIMID 25 mg daily.
Elimination
The mean half-life of lenalidomide is 3 hours in healthy subjects and 3 to 5 hours in patients with MM, MDS or MCL.
Metabolism
Lenalidomide undergoes limited metabolism. Unchanged lenalidomide is the predominant circulating component in humans. Two identified metabolites are 5-
hydroxy-lenalidomide and N-acetyl-lenalidomide; each constitutes less than 5% of parent levels in circulation.
Excretion
Elimination is primarily renal. Following a single oral administration of [
14
C]-lenalidomide 25 mg to healthy subjects, approximately 90% and 4% of the radioactive
dose was eliminated within ten days in urine and feces, respectively. Approximately 82% of the radioactive dose was excreted as lenalidomide in the urine within 24
hours. Hydroxy-lenalidomide and N-acetyl-lenalidomide represented 4.6% and 1.8% of the excreted dose, respectively. The renal clearance of lenalidomide exceeds
the glomerular filtration rate.
Specific Populations
Renal Impairment: Eight subjects with mild renal impairment (creatinine clearance (CLcr) 50 to 79 mL/min calculated using Cockcroft-Gault), 9 subjects with
moderate renal impairment (CLcr 30 to 49 mL/min), 4 subjects with severe renal impairment (CLcr < 30 mL/min), and 6 patients with end stage renal disease (ESRD)
requiring dialysis were administered a single 25 mg dose of REVLIMID. Three healthy subjects of similar age with normal renal function (CLcr > 80 mL/min) were
also administered a single 25 mg dose of REVLIMID. As CLcr decreased, half-life increased and drug clearance decreased linearly. Patients with moderate and severe
impairment had a 3-fold increase in half-life and a 66% to 75% decrease in drug clearance compared to healthy subjects. Patients on hemodialysis (n=6) had an
approximate 4.5-fold increase in half-life and an 80% decrease in drug clearance compared to healthy subjects. Approximately 30% of the drug in body was removed
during a 4-hour hemodialysis session.
Adjust the starting dose of REVLIMID in patients with renal impairment based on the CLcr value [see Dosage and Administration (2.5)].
Hepatic Impairment: Mild hepatic impairment (defined as total bilirubin > 1 to 1.5 times upper limit normal (ULN) or any aspartate transaminase greater than ULN) did
not influence the disposition of lenalidomide. No pharmacokinetic data is available for patients with moderate to severe hepatic impairment.
Other Intrinsic Factors: Age (39 to 85 years), body weight (33 to 135 kg), sex, race, and type of hematological malignancies (MM, MDS or MCL) did not have a
clinically relevant effect on lenalidomide clearance in adult patients.
Drug Interactions
Co-administration of a single dose or multiple doses of dexamethasone (40 mg) had no clinically relevant effect on the multiple dose pharmacokinetics of REVLIMID
(25 mg).
Co-administration of REVLIMID (25 mg) after multiple doses of a P-gp inhibitor such as quinidine (600 mg twice daily) did not significantly increase the Cmax or
AUC of lenalidomide.
Co-administration of the P-gp inhibitor and substrate temsirolimus (25 mg),with REVLIMID (25 mg) did not significantly alter the pharmacokinetics of lenalidomide,
temsirolimus, or sirolimus (metabolite of temsirolimus).
In vitro studies demonstrated that REVLIMID is a substrate of P-glycoprotein (P-gp). REVLIMID is not a substrate of human breast cancer resistance protein (BCRP),
multidrug resistance protein (MRP) transporters MRP1, MRP2, or MRP3, organic anion transporters (OAT) OAT1 and OAT3, organic anion transporting polypeptide
1B1 (OATP1B1), organic cation transporters (OCT) OCT1 and OCT2, multidrug and toxin extrusion protein (MATE) MATE1, and organic cation transporters novel
(OCTN) OCTN1 and OCTN2. Lenalidomide is not an inhibitor of P-gp, bile salt export pump (BSEP), BCRP, MRP2, OAT1, OAT3, OATP1B1, OATP1B3, or OCT2.
Lenalidomide does not inhibit or induce CYP450 isoenzymes. Also, lenalidomide does not inhibit bilirubin glucuronidation formation in human liver microsomes with
UGT1A1 genotyped as UGT1A1*1/*1, UGT1A1*1/*28, and UGT1A1*28/*28.
NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with lenalidomide have not been conducted.
Reference ID: 4439576
30
13
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
14
Lenalidomide was not mutagenic in the bacterial reverse mutation assay (Ames test) and did not induce chromosome aberrations in cultured human peripheral blood
lymphocytes, or mutations at the thymidine kinase (tk) locus of mouse lymphoma L5178Y cells. Lenalidomide did not increase morphological transformation in Syrian
Hamster Embryo assay or induce micronuclei in the polychromatic erythrocytes of the bone marrow of male rats.
A fertility and early embryonic development study in rats, with administration of lenalidomide up to 500 mg/kg (approximately 200 times the human dose of 25 mg,
based on body surface area) produced no parental toxicity and no adverse effects on fertility.
CLINICAL STUDIES
14.1 Multiple Myeloma
Randomized, Open-Label Clinical Trial in Patients with Newly Diagnosed MM:
A randomized multicenter, open-label, 3-arm trial of 1,623 patients, was conducted to compare the efficacy and safety of REVLIMID and low-dose dexamethasone
(Rd) given for 2 different durations of time to that of melphalan, prednisone and thalidomide (MPT) in newly diagnosed MM patients who were not a candidate for
stem cell transplant. In the first arm of the study, Rd was given continuously until progressive disease [Arm Rd Continuous]. In the second arm, Rd was given for up to
eighteen 28-day cycles [72 weeks, Arm Rd18]). In the third arm, melphalan, prednisone and thalidomide (MPT) was given for a maximum of twelve 42-day cycles (72
weeks). For the purposes of this study, a patient who was < 65 years of age was not a candidate for SCT if the patient refused to undergo SCT therapy or the patient did
not have access to SCT due to cost or other reasons. Patients were stratified at randomization by age (≤75 versus >75 years), stage (ISS Stages I and II versus Stage III),
and country.
Patients in the Rd Continuous and Rd18 arms received REVLIMID 25 mg once daily on Days 1 to 21 of 28-day cycles. Dexamethasone was dosed 40 mg once daily
on Days 1, 8, 15, and 22 of each 28-day cycle. For patients over > 75 years old, the starting dose of dexamethasone was 20 mg orally once daily on days 1,8,15, and 22
of repeated 28-day cycles. Initial dose and regimens for Rd Continuous and Rd18 were adjusted according to age and renal function. All patients received prophylactic
anticoagulation with the most commonly used being aspirin.
The demographics and disease-related baseline characteristics of the patients were balanced among the 3 arms. In general, study subjects had advanced-stage disease.
Of the total study population, the median age was 73 in the 3 arms with 35% of total patients > 75 years of age; 59% had ISS Stage I/II; 41% had ISS stage III; 9% had
severe renal impairment (creatinine clearance [CLcr] < 30 mL/min); 23% had moderate renal impairment (CLcr > 30 to 50 mL/min; 44% had mild renal impairment
(CLcr > 50 to 80 mL/min). For ECOG Performance Status, 29% were Grade 0, 49% Grade 1, 21% Grade 2, 0.4% ≥ Grade 3.
The primary efficacy endpoint, progression-free survival (PFS), was defined as the time from randomization to the first documentation of disease progression as
determined by Independent Response Adjudication Committee (IRAC), based on International Myeloma Working Group [IMWG] criteria or death due to any cause,
whichever occurred first during the study until the end of the PFS follow-up phase. For the efficacy analysis of all endpoints, the primary comparison was between Rd
Continuous and MPT arms. The efficacy results are summarized in the table below. PFS was significantly longer with Rd Continuous than MPT: HR 0.72 (95% CI:
0.61-0.85 p <0.0001). A lower percentage of subjects in the Rd Continuous arm compared with the MPT arm had PFS events (52% versus 61%, respectively). The
improvement in median PFS time in the Rd Continuous arm compared with the MPT arm was 4.3 months. The myeloma response rate was higher with Rd Continuous
compared with MPT (75.1% versus 62.3%); with a complete response in 15.1% of Rd Continuous arm patients versus 9.3% in the MPT arm. The median time to first
response was 1.8 months in the Rd Continuous arm versus 2.8 months in the MPT arm.
For the interim OS analysis with 03 March 2014 data cutoff, the median follow-up time for all surviving patients is 45.5 months, with 697 death events, representing
78% of prespecified events required for the planned final OS analysis (697/896 of the final OS events). The observed OS HR was 0.75 for Rd Continuous versus MPT
(95% CI = 0.62, 0.90).
Table 13: Overview of Efficacy Results – Study MM-020 (Intent-to-treat Population)
Rd Continuous
(N = 535)
Rd18
(N = 541)
MPT
(N = 547)
PFS – IRAC (months)
g
Number of PFS events 278 (52) 348 (64.3) 334 (61.1)
Median
a
PFS time, months (95% CI)
b
25.5 (20.7, 29.4) 20.7 (19.4, 22) 21.2 (19.3, 23.2)
HR [95% CI]
c
;
p
-value
d
Rd Continuous vs MPT 0.72 (0.61, 0.85);
<0.0001
Rd Continuous vs Rd18 0.70 (0.60, 0.82)
Rd18 vs MPT 1.03 (0.89, 1.20)
Overall Survival (months)
h
Number of Death events 208
(
38.9
)
228
(
42.1
)
261
(
47.7
)
Median
a
OS time, months
(
95% CI
)
b
58.9
(
56, NE
)
f
56.7
(
50.1, NE
)
48.5
(
44.2, 52
)
HR [95% CI]
c
Rd Continuous vs MPT 0.75 (0.62, 0.90)
Rd Continuous vs Rd18 0.91 (0.75, 1.09)
Rd18 vs MPT
0.83
(
0.69, 0.99
)
Res
p
onse Rate
e
–
IRAC, n (%)
g
CR 81
(
15.1
)
77
(
14.2
)
51
(
9.3
)
VGPR 152
(
28.4
)
154
(
28.5
)
103
(
18.8
)
PR 169 (31.6) 166 (30.7) 187 (34.2)
Overall response: CR, VGPR, or PR 402 (75.1) 397 (73.4) 341 (62.3)
CR = complete response; d = low-dose dexamethasone; HR = hazard ratio; IRAC = Independent Response Adjudication Committee; M = melphalan;
NE = not estimable; OS = overall survival; P = prednisone; PFS = progression-free survival; PR = partial response; R = REVLIMID; Rd
Reference ID: 4439576
31
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Continuous = Rd given until documentation of progressive disease; Rd18 = Rd given for ≤ 18 cycles; T = thalidomide; VGPR = very good partial response; vs
= versus.
a
The median is based on the Kaplan-Meier estimate.
b
The 95% Confidence Interval (CI) about the median.
c
Based on Cox proportional hazards model comparing the hazard functions associated with the indicated treatment arms.
d
The p-value is based on the unstratified log-rank test of Kaplan-Meier curve differences between the indicated treatment arms.
e
Best assessment of response during the treatment phase of the study.
f
Including patients with no response assessment data or whose only assessment was “response not evaluable.”
g
Data cutoff date = 24 May 2013.
h
Data cutoff date = 3 March 2014.
Reference ID: 4439576
32
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
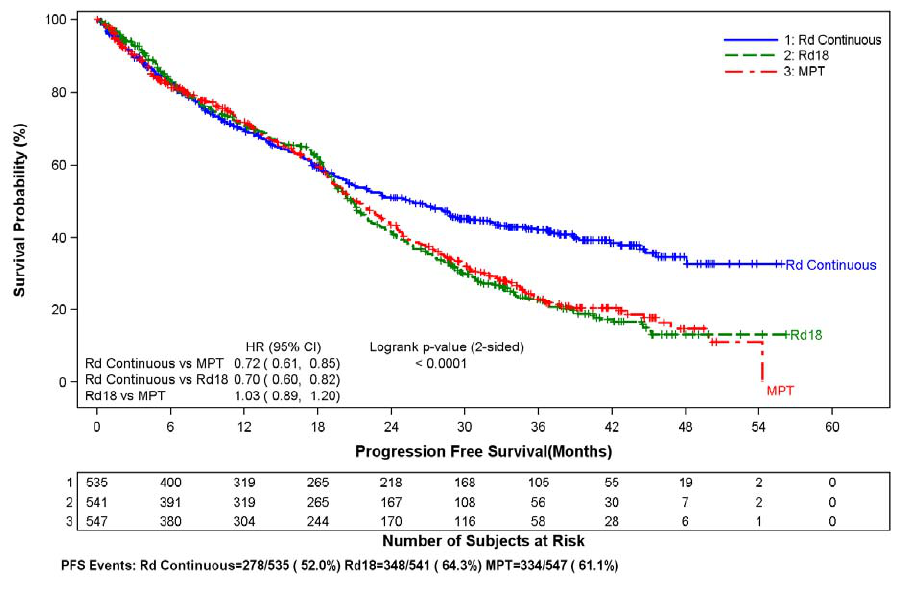
Kaplan-Meier Curves of Progression-free Survival Based on IRAC Assessment (ITT MM Population)
Between Arms Rd Continuous, Rd18 and MPT
Cutoff date: 24 May 2013
CI = confidence interval; d = low-dose dexamethasone; HR = hazard ratio; IRAC = Independent Response Adjudication Committee; M = melphalan; P = prednisone;
R = REVLIMID; Rd Continuous = Rd given until documentation of progressive disease; Rd18 = Rd given for ≤ 18 cycles; T = thalidomide.
Reference ID: 4439576
33
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
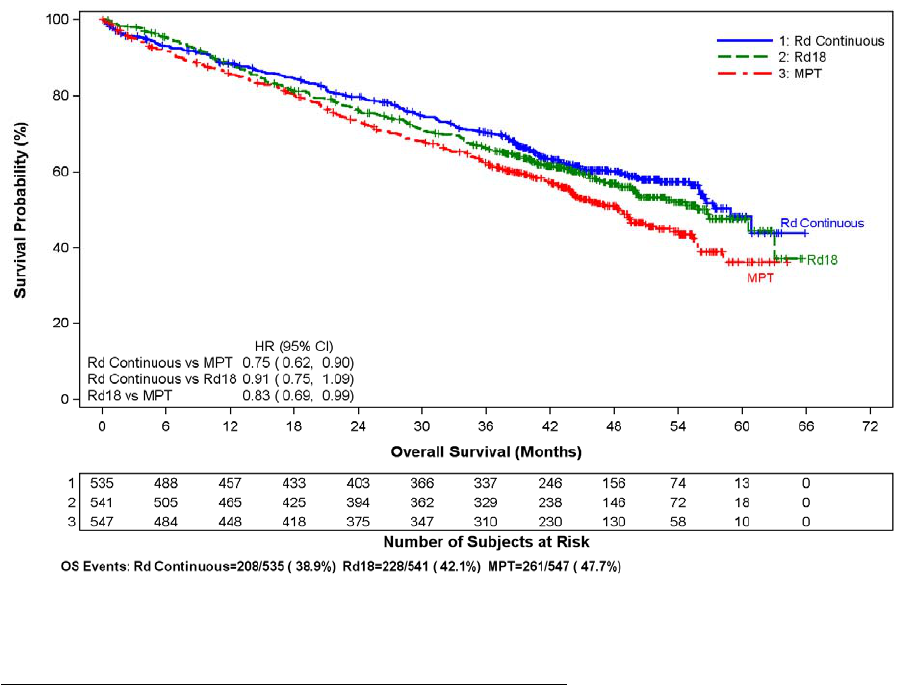
Kaplan-Meier Curves of Overall Survival (ITT MM Population)
Between Arms Rd Continuous, Rd18 and MPT
Cutoff date: 03 Mar 2014
CI = confidence interval; d = low-dose dexamethasone; HR = hazard ratio; M = melphalan; P = prednisone; R = REVLIMID; Rd Continuous = Rd given until
documentation of progressive disease; Rd18 = Rd given for ≤18 cycles; T = thalidomide.
Randomized, Placebo-Controlled Clinical Trials - Maintenance Following Auto-HSCT:
Two multicenter, randomized, double-blind, parallel group, placebo-controlled studies were conducted to evaluate the efficacy and safety of REVLIMID maintenance
therapy in the treatment of MM patients after auto-HSCT. In Maintenance Study 1, patients between 18 and 70 years of age who had undergone induction therapy
followed by auto-HSCT were eligible. Induction therapy must have occurred within 12 months. Within 90-100 days after auto-HSCT, patients with at least a stable
disease response were randomized 1:1 to receive either REVLIMID or placebo maintenance. In Maintenance Study 2, patients aged < 65 years at diagnosis who had
undergone induction therapy followed by auto-HSCT and had achieved at least a stable disease response at the time of hematologic recovery were eligible. Within 6
months after auto-HSCT, patients were randomized 1:1 to receive either REVLIMID or placebo maintenance. Patients eligible for both trials had to have CLcr
≥30 mL/minute.
In both studies, the REVLIMID maintenance dose was 10 mg once daily on days 1-28 of repeated 28-day cycles, could be increased to 15 mg once daily after 3 months
in the absence of dose-limiting toxicity, and treatment was to be continued until disease progression or patient withdrawal for another reason. The dose was reduced, or
treatment was temporarily interrupted or stopped, as needed to manage toxicity. A dose increase to 15 mg once daily occurred in 135 patients (58%) in Maintenance
Study 1, and in 185 patients (60%) in Maintenance Study 2.
The demographics and disease-related baseline characteristics of the patients were similar across the two studies and reflected a typical MM population after auto-
HSCT (see Table 14).
Reference ID: 4439576
34
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Table 14: Baseline Demographic and Disease-Related Characteristics – MM Maintenance Studies 1 and 2
Maintenance Study 1 Maintenance Study 2
REVLIMID
N = 231
Placebo
N = 229
REVLIMID
N = 307
Placebo
N = 307
Age (years)
Median 58 58 57.5 58.1
(Min, max) (29, 71) (39, 71) (22.7, 68.3) (32.3, 67)
Sex, n (%)
Male 121 (52) 129 (56) 169 (55) 181 (59)
Female 110 (48) 100 (44) 138 (45) 126 (41)
ISS Stage at Diagnosis,
n (%)
Stage I or II 120 (52) 131 (57) 232 (76) 250 (81)
Stage
I
62 (27) 85 (37) 128 (42) 143 (47)
Stage I
I
58 (25) 46 (20) 104 (34) 107 (35)
Stage III 39 (17) 35 (15) 66 (21) 46 (15)
Missing 72 (31) 63 (28) 9 (3) 11 (4)
CrCl at Post-auto-HSCT,
n (%)
< 50 mL/min
23 (10) 16 (7)
10 (3) 9 (3)
50 mL/min
201 (87) 204 (89) 178 (58) 200 (65)
Missing 7 (3) 9 (4) 119 (39) 98 (32)
Data cutoff date = 1 March 2015.
The major efficacy endpoint of both studies was PFS defined from randomization to the date of progression or death, whichever occurred first; the individual studies
were not powered for an overall survival endpoint. Both studies were unblinded upon the recommendations of their respective data monitoring committees and after
surpassing the respective thresholds for preplanned interim analyses of PFS. After unblinding, patients continued to be followed as before. Patients in the placebo arm
of Maintenance Study 1 were allowed to cross over to receive REVLIMID before disease progression (76 patients [33%] crossed over to REVLIMID); patients in
Maintenance Study 2 were not recommended to cross over. The efficacy results are summarized in the following table. In both studies, the primary analysis of PFS at
unblinding was significantly longer with REVLIMID compared to placebo: Maintenance Study 1 HR 0.38 (95% CI: 0.27-0.54 p <0.001) and Maintenance Study 2 HR
0.50 (95% CI: 0.39-0.64 p <0.001). For both studies, PFS was updated with a cutoff date of 1 March 2015 as shown in the table and the following Kaplan Meier graphs.
With longer follow-up (median 72.4 and 86.0 months, respectively), the updated PFS analyses for both studies continue to show a PFS advantage for REVLIMID
compared to placebo: Maintenance Study 1 HR 0.38 (95% CI: 0.28-0.50) with median PFS of 68.6 months and Maintenance Study 2 HR 0.53 (95% CI: 0.44-0.64) with
median PFS of 46.3 months.
Descriptive analysis of OS data with a cutoff date of 1 February 2016 are provided in Table 15. Median follow-up time was 81.6 and 96.7 months for
Maintenance Study 1 and Maintenance Study 2, respectively. Median OS was 111.0 and 84.2 months for REVLIMID and placebo, respectively, for Maintenance
Study 1, and 105.9 and 88.1 months, for REVLIMID and placebo, respectively, for Maintenance Study 2.
Reference ID: 4439576
35
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Table 15: Progression-free Survival and Overall Survival from
Randomization in MM Maintenance Studies 1 and 2 (ITT Post-Auto-HSCT Population)
Maintenance Study 1 Maintenance Study 2
REVLIMID
N = 231
Placebo
N = 229
REVLIMID
N = 307
Placebo
N = 307
PFS at Unblinding
PFS Events n (%)
46 (20) 98 (43) 103 (34) 160 (52)
Median in months [95% CI]
33.9
[NE, NE]
19
[16.2, 25.6]
41.2
[38.3, NE]
23.0
[21.2, 28.0]
Hazard Ratio
[95% CI]
0.38
[0.27, 0.54]
0.50
[0.39, 0.64]
Log-rank Test p-value
<0.001 <0.001
PFS at Updated Analysis
1 March 2015 (Studies 1 and 2)
PFS Events n (%) 97 (42) 116 (51) 191 (62) 248 (81)
Median in months [95% CI]
68.6
[52.8, NE]
22.5
[18.8, 30.0]
46.3
[40.1, 56.6]
23.8
[21.0, 27.3]
Hazard Ratio
[95% CI]
0.38
[0.28, 0.50]
0.53
[0.44, 0.64]
OS at Updated Analysis
1 Feb 2016 (Studies 1 and 2)
OS Events n (%) 82 (35) 114 (50) 143 (47) 160 (52)
Median in months [95% CI]
111
[101.8, NE]
84.2
[71.0, 102.7]
105.9
[88.8, NE]
88.1
[80.7, 108.4]
Hazard Ratio
[95% CI]
0.59
[0.44, 0.78]
0.90
[0.72, 1.13]
Date of Unblinding in Maintenance Study 1 and 2 = 17 December 2009 and 7 July 2010, respectively.
Auto-HSCT = autologous hematopoietic stem cell transplantation; CI = confidence interval; ITT = intent to treat; NE = not estimable;
PFS = progression-free survival.
PFS at time of unblinding for Maintenance Study 2 was based on assessment by an Independent Review Committee. All other PFS
analyses were based on assessment by investigator.
Note: The median is based on Kaplan-Meier estimate, with 95% CIs about the median overall PFS time. Hazard ratio is based on a
proportional hazards model stratified by stratification factors comparing the hazard functions associated with treatment arms
(REVLIMID:placebo).
Reference ID: 4439576
36
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
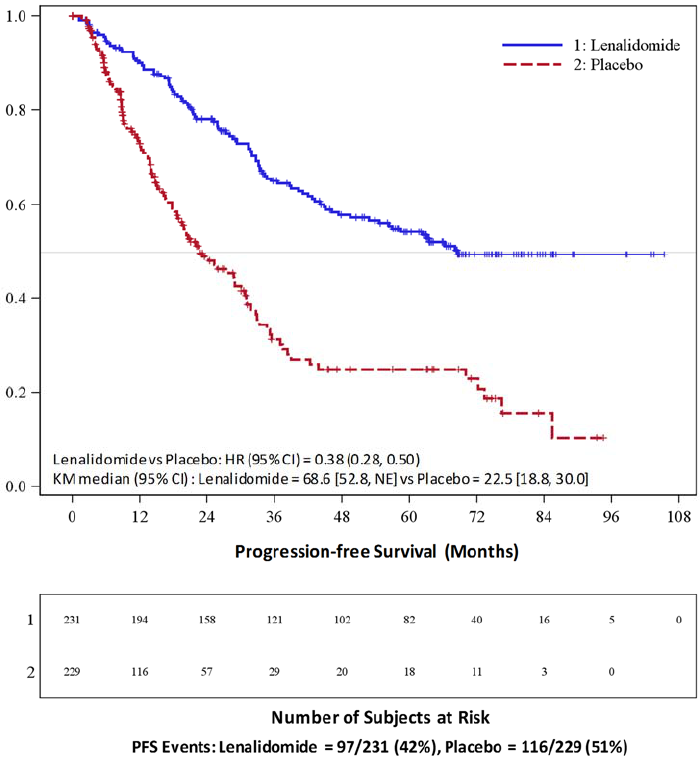
Kaplan-Meier Curves of Progression-free Survival from Randomization
(ITT Post-Auto-HSCT Population) in MM Maintenance Study 1 between REVLIMID and
Placebo Arms (Updated Cutoff Date 1 March 2015)
Auto-HSCT = autologous hematopoietic stem cell transplantation; CI = confidence interval; HR = hazard ratio; ITT = intent to treat; KM = Kaplan-Meier;
PFS = progression-free survival; vs = versus.
Reference ID: 4439576
37
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
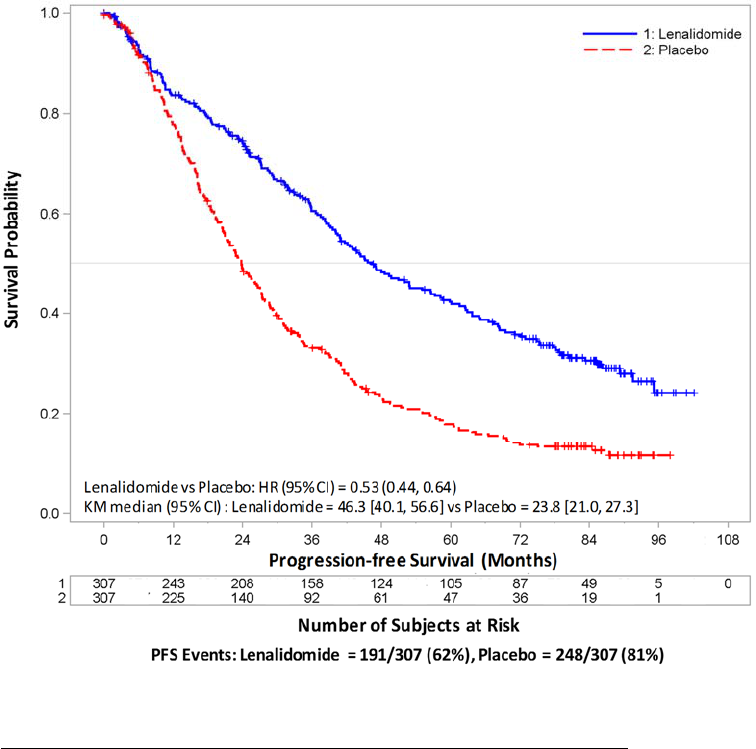
Kaplan-Meier Curves of Progression-free Survival from Randomization
(ITT Post-Auto-HSCT Population) in MM Maintenance Study 2 between
REVLIMID and Placebo Arms (Updated Cutoff Date 1 March 2015)
Auto-HSCT = autologous hematopoietic stem cell transplantation; CI = confidence interval; HR = hazard ratio; ITT = intent to treat; KM = Kaplan-Meier; NE = not
estimable; PFS = progression-free survival; vs = versus.
Randomized, Open-Label Clinical Studies in Patients with MM After At Least One Prior Therapy
Two randomized studies (Studies 1 and 2) were conducted to evaluate the efficacy and safety of REVLIMID. These multicenter, multinational, double-blind, placebo-
controlled studies compared REVLIMID plus oral pulse high-dose dexamethasone therapy to dexamethasone therapy alone in patients with MM who had received at
least one prior treatment. These studies enrolled patients with absolute neutrophil counts (ANC) ≥ 1000/mm
3
, platelet counts ≥ 75,000/mm
3
, serum creatinine ≤ 2.5
mg/dL, serum SGOT/AST or SGPT/ALT ≤ 3 x upper limit of normal (ULN), and serum direct bilirubin ≤ 2 mg/dL.
In both studies, patients in the REVLIMID/dexamethasone group took 25 mg of REVLIMID orally once daily on Days 1 to 21 and a matching placebo capsule once
daily on Days 22 to 28 of each 28-day cycle. Patients in the placebo/dexamethasone group took 1 placebo capsule on Days 1 to 28 of each 28-day cycle. Patients in both
treatment groups took 40 mg of dexamethasone orally once daily on Days 1 to 4, 9 to 12, and 17 to 20 of each 28-day cycle for the first 4 cycles of therapy.
The dose of dexamethasone was reduced to 40 mg orally once daily on Days 1 to 4 of each 28-day cycle after the first 4 cycles of therapy. In both studies, treatment was
to continue until disease progression.
In both studies, dose adjustments were allowed based on clinical and laboratory findings. Sequential dose reductions to 15 mg daily, 10 mg daily and 5 mg daily were
allowed for toxicity [see Dosage and Administration (2.1)].
Table 16 summarizes the baseline patient and disease characteristics in the two studies. In both studies, baseline demographic and disease-related characteristics were
comparable between the REVLIMID/dexamethasone and placebo/dexamethasone groups.
Reference ID: 4439576
38
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
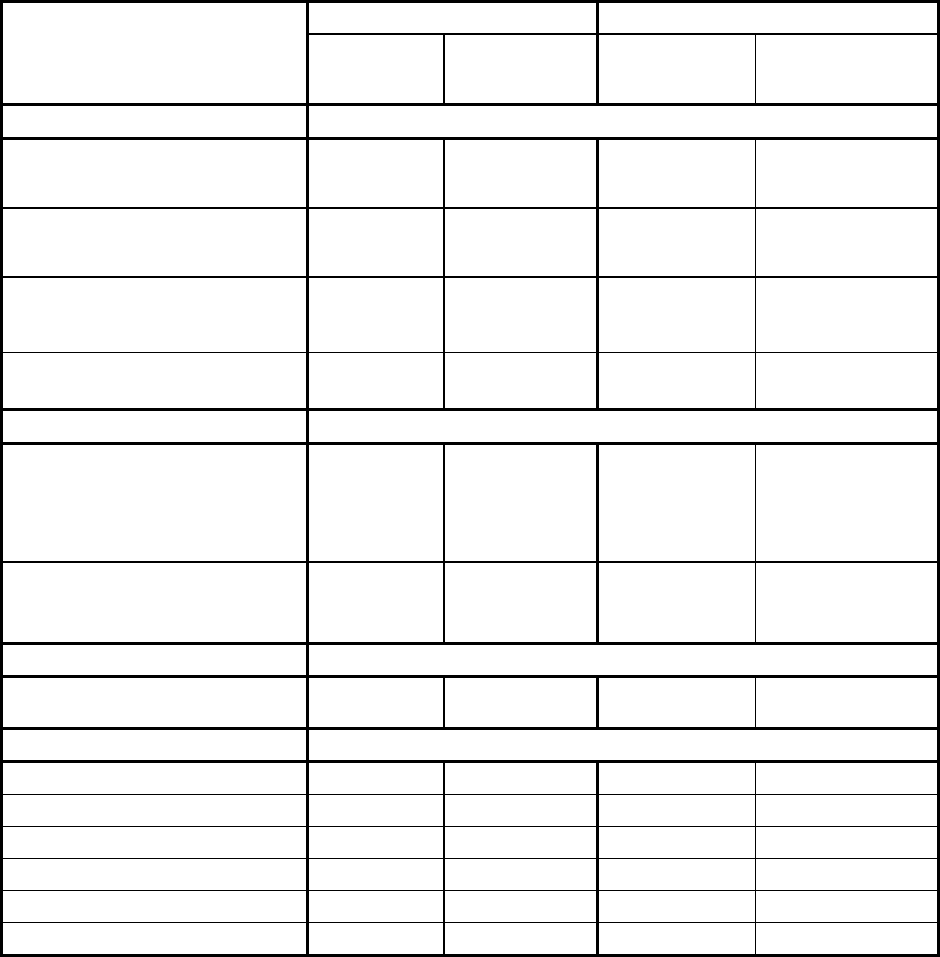
Table 16: Baseline Demographic and Disease-Related Characteristics – MM Studies 1 and 2
Study 1 Study 2
REVLIMID/Dex
N=177
Placebo/Dex
N=176
REVLIMID/Dex
N=176
Placebo/Dex
N=175
Patient Characteristics
Age (years)
Median
Min, Max
64
36, 86
62
37, 85
63
33, 84
64
40, 82
Sex
Male
Female
106 (60%)
71 (40%)
104 (59%)
72 (41%)
104 (59%)
72 (41%)
103 (59%)
72 (41%)
Race/Ethnicity
White
Other
141(80%)
36 (20%)
148 (84%)
28 (16%)
172 (98%)
4 (2%)
175 (100%)
0 (0%)
ECOG Performance
Status 0-1 157 (89%) 168 (95%) 150 (85%) 144 (82%)
Disease Characteristics
Multiple Myeloma Stage (Durie-Salmon)
I
II
III
3%
32%
64%
3%
31%
66%
6%
28%
65%
5%
33%
63%
β2-microglobulin (mg/L)
≤ 2.5 mg/L
> 2.5 mg/L
52 (29%)
125 (71%)
51 (29%)
125 (71%)
51 (29%)
125 (71%)
48 (27%)
127 (73%)
Number of Prior Therapies
1
≥ 2
38%
62%
38%
62%
32%
68%
33%
67%
Types of Prior Therapies
Stem Cell Transplantation 62% 61% 55% 54%
Thalidomide 42% 46% 30% 38%
Dexamethasone 81% 71% 66% 69%
Bortezomib 11% 11% 5% 4%
Melphalan 33% 31% 56% 52%
Doxorubicin 55% 51% 56% 57%
The primary efficacy endpoint in both studies was time to progression (TTP). TTP was defined as the time from randomization to the first occurrence of progressive
disease.
Preplanned interim analyses of both studies showed that the combination of REVLIMID/dexamethasone was significantly superior to dexamethasone alone for TTP.
The studies were unblinded to allow patients in the placebo/dexamethasone group to receive treatment with the REVLIMID/dexamethasone combination. For both
studies, the extended follow-up survival data with crossovers were analyzed. In study 1, the median survival time was 39.4 months (95%CI: 32.9, 47.4) in
REVLIMID/dexamethasone group and 31.6 months (95% CI: 24.1, 40.9) in placebo/dexamethasone group, with a hazard ratio of 0.79 (95% CI: 0.61-1.03). In study 2,
the median survival time was 37.5 months (95%CI: 29.9, 46.6) in REVLIMID/dexamethasone group and 30.8 months (95%CI: 23.5, 40.3) in placebo/dexamethasone
group, with a hazard ratio of 0.86 (95% CI: 0.65-1.14).
Reference ID: 4439576
39
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
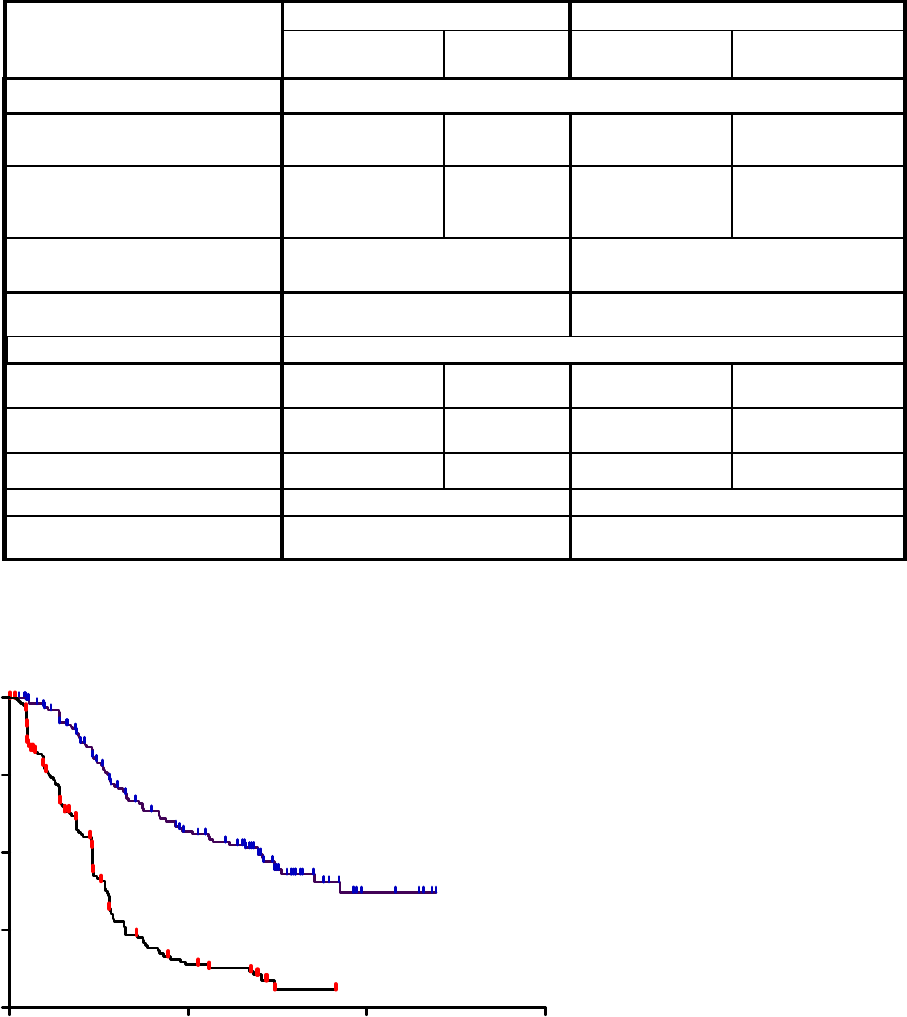
Table 17: TTP Results in MM Study 1 and Study 2
Study 1 Study 2
REVLIMID/Dex
N=177
Placebo/Dex
N=176
REVLIMID/Dex
N=176
Placebo/Dex
N=175
TTP
Events n
(
%
)
73
(
41
)
120
(
68
)
68
(
39
)
130
(
74
)
Median TTP in months [95% CI]
13.9
[9.5, 18.5]
4.7
[3.7, 4.9]
12.1
[9.5, NE]
4.7
[3.8, 4.8]
Hazard Ratio
[95% CI]
0.285
[0.210, 0.386]
0.324
[0.240, 0.438]
Log-rank Test p-value
3
<0.001 <0.001
Response
Complete Response (CR) n (%)
23 (13) 1 (1) 27 (15) 7 (4)
Partial Response (RR/PR) n (%)
84 (48) 33 (19) 77 (44) 34 (19)
Overall Response n (%)
107 (61) 34 (19) 104 (59) 41 (23)
p-value <0.001 <0.001
Odds Ratio [95% CI] 6.38
[3.95, 10.32]
4.72
[2.98, 7.49]
Kaplan-Meier Estimate of Time to Progression — MM Study 1
0 10 20 30
0
25
50
75
100
Revlimid/Dex
Placebo/Dex
Log Rank p < 0.001
HR (95% CI) = 0.285 (0.210-0.386)
Proportion of Subjects
Time to Progression (months)
Reference ID: 4439576
40
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
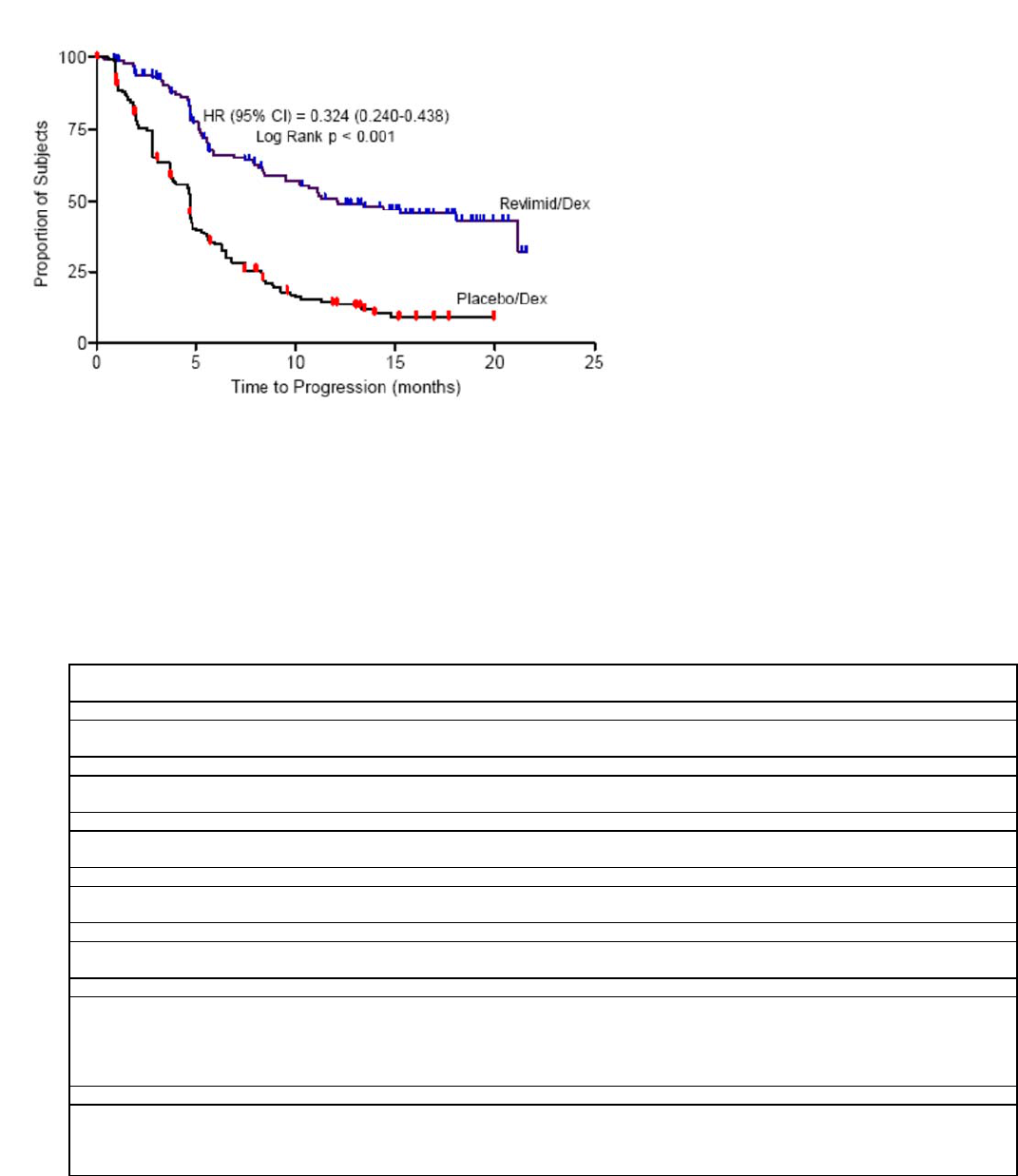
Kaplan-Meier Estimate of Time to Progression — MM Study 2
14.2 Myelodysplastic Syndromes (MDS) with a Deletion 5q Cytogenetic Abnormality
The efficacy and safety of REVLIMID were evaluated in patients with transfusion-dependent anemia in low- or intermediate-1- risk MDS with a 5q (q31-33)
cytogenetic abnormality in isolation or with additional cytogenetic abnormalities, at a dose of 10 mg once daily or 10 mg once daily for 21 days every 28 days in an
open-label, single-arm, multi-center study. The major study was not designed nor powered to prospectively compare the efficacy of the 2 dosing regimens. Sequential
dose reductions to 5 mg daily and 5 mg every other day, as well as dose delays, were allowed for toxicity [Dosage and Administration (2.2)].
This major study enrolled 148 patients who had RBC transfusion dependent anemia. RBC transfusion dependence was defined as having received ≥ 2 units of RBCs
within 8 weeks prior to study treatment. The study enrolled patients with absolute neutrophil counts (ANC) ≥ 500/mm
3
, platelet counts ≥ 50,000/mm
3
, serum creatinine
≤ 2.5 mg/dL, serum SGOT/AST or SGPT/ALT ≤ 3 x upper limit of normal (ULN), and serum direct bilirubin ≤ 2 mg/dL. Granulocyte colony-stimulating factor was
permitted for patients who developed neutropenia or fever in association with neutropenia. Baseline patient and disease-related characteristics are summarized in
Table 18.
Table 18: Baseline Demographic and Disease-Related Characteristics in the MDS Study
Overall
(N=148)
Age (years)
Median
Min, Max
71
37, 95
Gender n (%)
Male
Female
51
97
(34.5)
(65.5)
Race n (%)
White
Other
143
5
(96.6)
( 3.4)
Duration of MDS (years)
Median
Min, Max
2.5
0.1, 20.7
Del 5 (q31-33) Cytogenetic Abnormality n (%)
Yes
Other cytogenetic abnormalities
148
37
(100)
(25.2)
IPSS Score
a
n (%)
Low (0)
Intermediate-1 (0.5-1.0)
Intermediate-2 (1.5-2.0)
High (≥2.5)
Missing
55
65
6
2
20
(37.2)
(43.9)
( 4.1)
( 1.4)
(13.5)
FAB Classification
b
from central review n (%)
RA
RARS
RAEB
CMML
77
16
30
3
(52)
(10.8)
(20.3)
( 2)
a
IPSS Risk Category: Low (combined score = 0), Intermediate-1 (combined score = 0.5 to 1),
Intermediate-2 (combined score = 1.5 to 2.0), High (combined score ≥ 2.5); Combined score =
(Marrow blast score + Karyotype score + Cytopenia score).
b
French-American-British (FAB) classification of MDS.
The frequency of RBC transfusion independence was assessed using criteria modified from the International Working Group (IWG) response criteria for MDS. RBC
transfusion independence was defined as the absence of any RBC transfusion during any consecutive “rolling” 56 days (8 weeks) during the treatment period.
Reference ID: 4439576
41
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Transfusion independence was seen in 99/148 (67%) patients (95% CI [59, 74]). The median duration from the date when RBC transfusion independence was first
declared (i.e., the last day of the 56-day RBC transfusion-free period) to the date when an additional transfusion was received after the 56-day transfusion-free period
among the 99 responders was 44 weeks (range of 0 to >67 weeks). Ninety percent of patients who achieved a transfusion benefit did so by completion of three months
in the study.
RBC transfusion independence rates were unaffected by age or gender.
The dose of REVLIMID was reduced or interrupted at least once due to an adverse event in 118 (79.7%) of the 148 patients; the median time to the first dose reduction
or interruption was 21 days (mean, 35.1 days; range, 2-253 days), and the median duration of the first dose interruption was 22 days (mean, 28.5 days; range, 2-265
days). A second dose reduction or interruption due to adverse events was required in 50 (33.8%) of the 148 patients. The median interval between the first and second
dose reduction or interruption was 51 days (mean, 59.7 days; range, 15-205 days) and the median duration of the second dose interruption was 21 days (mean, 26 days;
range, 2-148 days).
14.3 Mantle Cell Lymphoma
A multicenter, single-arm, open-label trial of single-agent REVLIMID was conducted to evaluate the safety and efficacy of REVLIMID in patients with mantle cell
lymphoma who have relapsed after or were refractory to bortezomib or a bortezomib-containing regimen. Patients with a creatinine clearance ≥60 mL/min were given
REVLIMID at a dose of 25 mg once daily for 21 days every 28 days. Patients with a creatinine clearance ≥30 mL/min and <60 mL/min were given REVLIMID at a
dose of 10 mg once daily for 21 days every 28 days. Treatment was continued until disease progression, unacceptable toxicity, or withdrawal of consent.
The trial included patients who were at least 18 years of age with biopsy-proven MCL with measurable disease by CT scan. Patients were required to have received
prior treatment with an anthracycline or mitoxantrone, cyclophosphamide, rituximab, and bortezomib, alone or in combination. Patients were required to have
documented refractory disease (defined as without any response of PR or better during treatment with bortezomib or a bortezomib-containing regimen), or relapsed
disease (defined as progression within one year after treatment with bortezomib or a bortezomib-containing regimen). At enrollment patients were to have an absolute
neutrophil counts (ANC) ≥1500/ mm
3
, platelet counts ≥ 60,000/mm
3
, serum SGOT/AST or SGPT/ALT ≤3x upper limit of normal (ULN) unless there was documented
evidence of liver involvement by lymphoma, serum total bilirubin ≤1.5 x ULN except in cases of Gilbert’s syndrome or documented liver involvement by lymphoma,
and calculated creatinine clearance (Cockcroft-Gault formula) ≥30 mL/min.
The median age was 67 years (43-83), 81% were male and 96% were Caucasian. The table below summarizes the baseline disease-related characteristics and prior anti-
lymphoma therapy in the Mantle Cell Lymphoma trial.
Table 19: Baseline Disease-related Characteristics and Prior Anti –Lymphoma Therapy in
Mantle Cell Lymphoma Trial
Baseline Disease Characteristics and Prior Anti -
Lymphoma Treatment
Total Patients
(N=134)
ECOG Performance Status
a
n (%)
0
1
2
3
43 (32)
73 (54)
17 (13)
1 (<1)
Advanced MCL Stage, n (%)
III
IV
27 (20)
97 (72)
High or Intermediate MIPI Score
b
, n (%) 90 (67)
High Tumor Burden
c
, n (%) 77 (57)
Bulky Disease
d
, n (%) 44 (33)
Extranodal Disease, n (%) 101 (75)
Number of Prior Systemic Anti-Lymphoma
Therapies, n (%)
Median (range)
1
2
3
≥ 4
4 (2, 10)
0 (0)
29 (22)
34 (25)
71 (53)
Number of Subjects Who Received Prior Regimen
Containing, n (%):
Anthracycline/mitoxantrone
Cyclophosphamide
Rituximab
Bortezomib
133 (99)
133 (99)
134 (100)
134 (100)
Refractory to Prior Bortezomib, n (%) 81 (60)
Refractory to Last Prior Therapy, n (%) 74 (55)
Prior Autologous Bone Marrow or Stem Cell
Transplant, n (%)
39 (29)
a
ECOG = Eastern Cooperative Oncology Group.
b
MIPI = MCL International Prognostic Index.
c
High tumor burden is defined as at least one lesion that is ≥5 cm in diameter or 3 lesions that are ≥3 cm in diameter.
d
Bulky disease is defined as at least one lesion that is ≥7cm in the longest diameter.
The efficacy endpoints in the MCL trial were overall response rate (ORR) and duration of response (DOR). Response was determined based on review of radiographic
scans by an independent review committee according to a modified version of the International Workshop Lymphoma Response Criteria (Cheson, 1999). The DOR is
defined as the time from the initial response (at least PR) to documented disease progression. The efficacy results for the MCL population were based on all evaluable
patients who received at least one dose of study drug and are presented in Table 20. The median time to response was 2.2 months (range 1.8 to 13 months).
Reference ID: 4439576
42
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Table 20: Response Outcomes in the Pivotal Mantle Cell Lymphoma Trial
Response Analyses (N = 133) N (%) 95% CI
Overall Response Rate (IWRC) (CR + CRu +PR) 34 (26) (18.4, 33.9)
Complete Response (CR + CRu) 9 (7) (3.1, 12.5)
CR 1 (1)
CRu 8 (6)
Partial Response (PR) 25 (19)
Duration of Response (months) Median 95% CI
Duration of Overall Response (CR + CRu + PR) (N = 34) 16.6 (7.7, 26.7)
14.4 Follicular and Marginal Zone Lymphoma
The efficacy of REVLIMID with rituximab in patients with relapsed or refractory follicular and marginal zone lymphoma was evaluated in the AUGMENT
(NCT01938001) and MAGNIFY (NCT01996865) trials.
AUGMENT is a randomized, double-blind, multicenter trial (n=358) in which patients with relapsed or refractory follicular or marginal zone lymphoma were
randomized 1:1 to receive REVLIMID and rituximab or rituximab and placebo. AUGMENT included patients diagnosed with Grade 1, 2, or 3a follicular lymphoma,
who received at least 1 prior systemic therapy, were refractory or relapsed, not rituximab-refractory, had at least one measurable nodal or extranodal lesion by CT or
MRI scan, and had adequate bone marrow, liver, and renal function. Randomization was stratified by follicular versus marginal zone lymphoma, previous rituximab
therapy, and time since other anti-lymphoma therapy. In AUGMENT, REVLIMID was administered orally 20 mg once daily for Days 1 to 21 of repeating 28-day
cycles for a maximum of 12 cycles or until unacceptable toxicity. The dose of rituximab was 375 mg/m
2
every week in Cycle 1 (Days 1, 8, 15, and 22) and on Day 1 of
every 28-day cycle from Cycles 2 through 5. All dosage calculations for rituximab were based on the patient’s body surface area (BSA), using actual patient weight.
Dose adjustments for REVLIMID were allowed based on clinical and laboratory findings. A patient with moderate renal insufficiency (≥30 to <60 mL/minute) received
a lower REVLIMID starting dose of 10 mg daily on the same schedule. After 2 cycles, the REVLIMID dose could be increased to 15 mg once daily on Days 1 to 21 of
each 28-day cycle if the patient tolerated the medication.
MAGNIFY is an open-label, multicenter trial (n=232) in which patients with relapsed or refractory follicular, marginal zone, or mantle cell lymphoma received 12
induction cycles of REVLIMID and rituximab. MAGNIFY included patients diagnosed with Grade 1, 2,3a, 3b follicular (including transformed), marginal zone, or
mantle cell lymphoma Stage I to IV who were previously treated for their lymphoma, had been refractory or had a relapse after their last treatment, had at least one
measurable nodal or extranodal lesion by CT or MRI scan, and had adequate bone marrow, liver, and renal function. Patients refractory to rituximab were also included.
The information from the subjects who received at least 1 dose of initial therapy in the first 12 induction cycles (n=222) in the MAGNIFY trial was included in the
evaluation of the efficacy of REVLIMID/rituximab in patients with relapsed or refractory follicular and marginal zone lymphoma. In MAGNIFY, REVLIMID 20 mg
was given on Days 1-21 of repeated 28-day cycles for up to 12 cycles or until unacceptable toxicity, progression, or withdrawal of consent. The dose of rituximab was
375 mg/m
2
every week in Cycle 1 (Days 1, 8, 15, and 22) and on Day 1 of every other 28-day cycle (Cycles 3,5,7,9, and 11) up to 12 cycles therapy. All dosage
calculations for rituximab were based on the patient BSA and actual weight. Dose adjustments were allowed based on clinical and laboratory findings.
The demographic and disease-related baseline characteristics in the AUGMENT and MAGNIFY trials are shown in the following table.
Table 21: Baseline Demographics and Disease-Related Characteristics of Patients with FL and MZL in AUGMENT and MAGNIFY Trials
Parameter
AUGMENT Trial MAGNIFY Trial
REVLIMID + Rituximab
(N=178)
Rituximab + Placebo
(Control Arm)
(N=180)
REVLIMID + Rituximab
(N=222)
Age (years)
Median (Max, Min) 64 (26, 86) 62 (35, 88) 65 (35, 91)
Age distribution, n (%)
<65 years 96 (54) 107 (59) 103 (46)
≥65 years 82 (46) 73 (41) 119 (54)
Sex, n (%)
Male 75 (42) 97 (54) 122 (55)
Female 103 (58) 83 (46) 100 (45)
Race
White 118 (66) 115 (64) 206 (93)
Other races 54 (30) 64 (36) 14 (6)
Not collected or reported 6 (3) 1 (0.6) 2 (1)
Body Surface Area (BSA,
m
2
)
Median (Max, Min) 1.8 (1.4, 3.1) 1.8 (1.3, 2.7) 2 (1.3, 2.6)
Disease Type FL or MZL
Follicular lymphoma 147 (83) 148 (82) 177 (80)
Marginal zone lymphoma 31 (17) 32 (18) 45 (20)
MZL subtype at diagnosis (investigator), n (%)
MALT 14 (45) 16 (50) 10 (22)
Nodal 8 (26) 10 (31) 25 (56)
Splenic 9 (29) 6 (19 10 (22)
FL stage at diagnosis (investigator), n (%)
FL Grade 1-2 125 (85) 123 (83) 149 (84)
FL Grade 3a 22 (15) 25 (17) 28 (16)
FLIPI score at baseline (calculated), n (%) Not Collected
Reference ID: 4439576
43
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Parameter
AUGMENT Trial MAGNIFY Trial
REVLIMID + Rituximab
(N=178)
Rituximab + Placebo
(Control Arm)
(N=180)
REVLIMID + Rituximab
(N=222)
Low risk (0,1) 52 (29) 67 (37)
Intermediate risk (2) 55 (31) 58 (32)
High risk (≥3) 69 (39) 54 (30)
Missing 2 (1) 1 (0.6)
ECOG score at baseline, n (%)
0 116 (65) 128 (71) 102 (46)
1 60 (34) 50 (28) 113 (51)
2 2 (1) 2 (1) 7 (3)
High tumor burden
b
at baseline, n (%)
Yes 97 (54) 86 (48) 148 (67)
No 81 (46) 94 (52) 74 (33)
Number of prior systemic antilymphoma
therapies
1 102 (57) 97 (54) 94 (42)
>1 76 (43) 83 (46) 128 (58)
Data Cutoff: 22 June 2018 (AUGMENT) and 1 May 2017 (MAGNIFY).
a
Patient had either 0 (n=2) or 1 prior systemic therapy.
b
Defined by GELF criteria.
ECOG = Eastern Cooperative Oncology Group; FLIPI = follicular lymphoma international prognostic index
In AUGMENT, efficacy was established in the intent-to-treat (ITT) population based on progression-free survival by Independent Review Committee using modified
2007 International Working Group response criteria. Efficacy results are summarized in Table 22.
Table 22: Efficacy Results for Patients in the AUGMENT Trial (ITT FL and MZL Population)
Parameter REVLIMID + Rituximab
(N=178)
Rituximab + Placebo
(N=180)
PFS
Patients with event, n (%) 68 (38.2) 115 (63.9)
Death 6 (8.8) 2 (1.7)
Progression of disease 62 (91.2) 113 (98.3)
PFS, median
a
[95% CI] (months) 39.4 [ 22.9, NE] 14.1 [11.4, 16.7]
HR
b
[95% CI] 0.46 [ 0.34, 0.62]
p-value
c
<0.0001
Objective response (CR+PR) , n(%)
[95% CI]
d
138 (77.5) [70.7, 83.4] 96 (53.3) [45.8, 60.8]
a
Median estimate is from Kaplan-Meier analysis.
b
hazard ratio and its CI were estimated from Cox proportional hazard model adjusting for the stratification 3: previous rituximab treatment
(yes, no), time since last antilymphoma therapy (≤ 2, > 2 years), and disease histology (FL, MZL).
c
p-value from log-rank test stratified by 3 factors noted above: previous rituximab treatment (yes, no), time since last antilymphoma therapy
(≤ 2, > 2 years), and disease histology (FL, MZL).
d
Exact confidence interval for binomial distribution.
Reference ID: 4439576
44
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
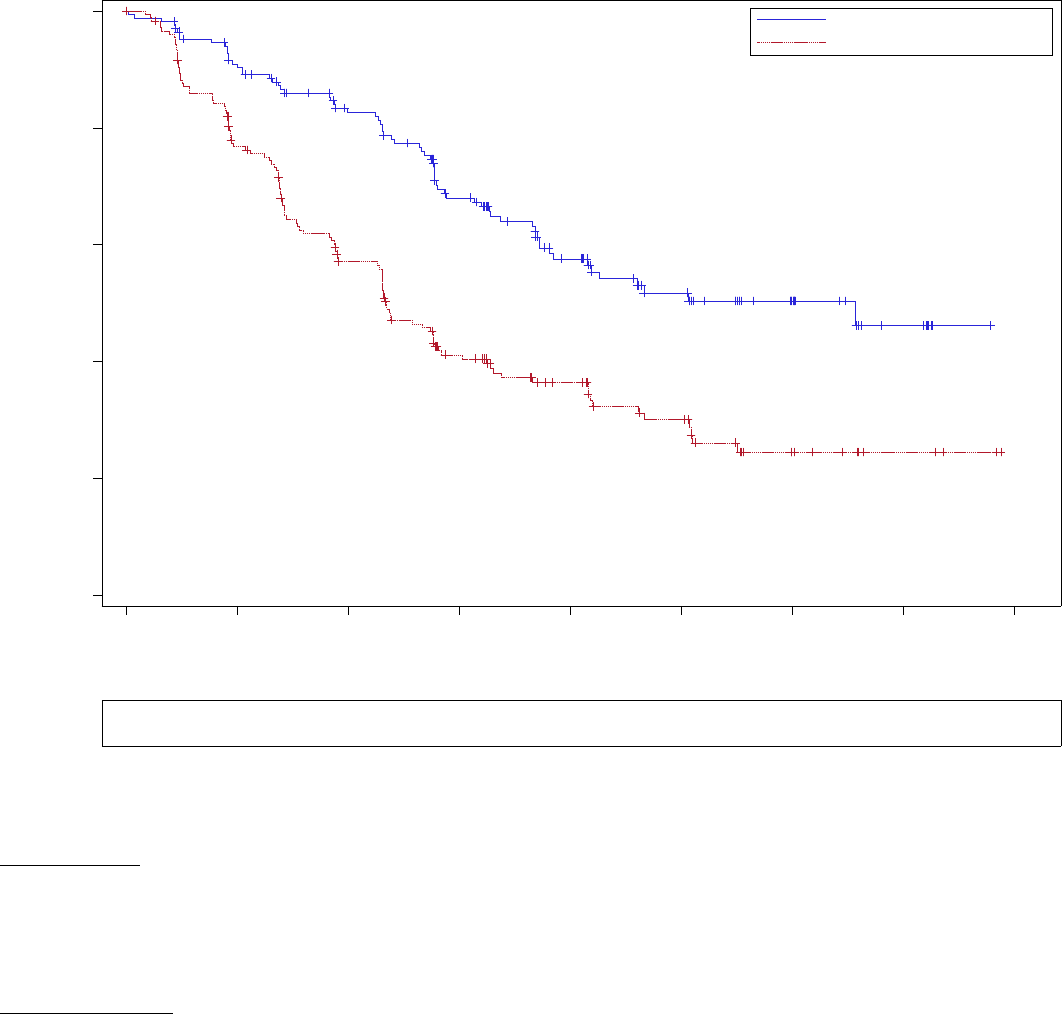
Kaplan-Meier Curves of Progression-free Survival by IRC Assessment Between Arms in AUGMENT Trial (ITT FL and MZL Population)
SSuurrvvivivaall PPrroobbaabbilitilityy
1.1.00
0.0.88
0.0.66
0.0.44
0.0.22
0.0.00
2: Rituximab + Placebo
1: Rituximab + Lenalidomide
Log Rank p[a] <0.0001
HR (95% CI)[ a] = 0.46 ( 0.34, 0.62)
00 66 1212 1818 2424 3030 3636 4242
PrProoggrreessssiioonn-- ffrreeee SuSurrvviivvaall ((MM oonntthhss))
1 178 148 124 91 59 39 20 7 0
2
180 132 92 58 40 26 10 4 0
N umber of Subj ects at Risk
a = Stratification factors included: previous rituximab treatment (y/n), time since last anti-lymphoma therapy (≤2 years, >2years), and disease histology (FL or MZL).
CI = confidence interval; HR = hazard ratio; KM = Kaplan-Meier; PFS = progression-free survival
Follicular Lymphoma
In AUGMENT, the objective response by IRC assessment for patients with follicular lymphoma was 80% (118/147) [95% CI: 73%, 86%]) in REVLIMID with
rituximab arm compared to 55% (82/148) [95% CI: 47, 64] in control arm.
In MAGNIFY, the overall response by investigator assessment was 59% (104/177) [95% CI: 51, 66] for patients with follicular lymphoma. Median duration of response
was not reached with a median follow-up time of 7.9 months [95% CI: 4.6, 9.2].
Marginal Zone Lymphoma
In AUGMENT, the objective response by IRC assessment for patients with marginal zone lymphoma was 65% (20/31) [95% CI: 45%, 81%] in REVLIMID with
rituximab arm compared to 44% (14/32) [95% CI: 26%, 62%] in control arm.
In MAGNIFY, the overall response by investigator assessment was 51% (23/45) [95% CI: 36, 66] for patients with marginal zone lymphoma. Median duration of
response was not reached with a median follow-up time of 11.5 months [95% CI: 8.0, 18.9].
15 REFERENCES
1. OSHA Hazardous Drugs. OSHA [Accessed on 29 January 2013, from http://www.osha.gov/SLTC/hazardousdrugs/index.html]
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
White and blue-green opaque hard capsules imprinted “REV” on one half and “2.5 mg” on the other half in black ink:
2.5 mg bottles of 28 (NDC 59572-402-28)
Reference ID: 4439576
45
4848
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
2.5 mg bottles of 100 (NDC 59572-402-00)
White opaque capsules imprinted “REV” on one half and “5 mg” on the other half in black ink:
5 mg bottles of 28 (NDC 59572-405-28)
5 mg bottles of 100 (NDC 59572-405-00)
Blue/green and pale yellow opaque capsules imprinted “REV” on one half and “10 mg” on the other half in black ink:
10 mg bottles of 28 (NDC 59572-410-28)
10 mg bottles of 100 (NDC 59572-410-00)
Powder blue and white opaque capsules imprinted “REV” on one half and “15 mg” on the other half in black ink:
15 mg bottles of 21 (NDC 59572-415-21)
15 mg bottles of 100 (NDC 59572-415-00)
Powder blue and blue-green opaque hard capsules imprinted “REV” on one half and “20 mg” on the other half in black ink.
20 mg bottles of 21 (NDC 59572-420-21)
20 mg bottles of 100 (NDC 59572-420-00)
White opaque capsules imprinted “REV” on one half and “25 mg” on the other half in black ink:
25 mg bottles of 21 (NDC 59572-425-21)
25 mg bottles of 100 (NDC 59572-425-00)
16.2 Storage
Store at 20°C - 25°C (68°F - 77°F); excursions permitted to 15°C - 30°C (59°F - 86°F) [See USP Controlled Room Temperature].
16.3 Handling and Disposal
Care should be exercised in the handling of REVLIMID. REVLIMID capsules should not be opened or broken. If powder from REVLIMID contacts the skin, wash the
skin immediately and thoroughly with soap and water. If REVLIMID contacts the mucous membranes, flush thoroughly with water.
Procedures for the proper handling and disposal of anticancer drugs should be considered. Several guidelines on the subject have been published.
1
Dispense no more than a 28-day supply.
Reference ID: 4439576
46
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved Patient labeling (Medication Guide)
Embryo-Fetal Toxicity
Advise patients that REVLIMID is contraindicated in pregnancy [see Boxed Warning and Contraindications (4.1)]. REVLIMID is a thalidomide analogue and can
cause serious birth defects or death to a developing baby [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
Advise females of reproductive potential that they must avoid pregnancy while taking REVLIMID and for at least 4 weeks after completing therapy.
Initiate REVLIMID treatment in females of reproductive potential only following a negative pregnancy test.
Advise females of reproductive potential of the importance of monthly pregnancy tests and the need to use 2 different forms of contraception including at least 1
highly effective form, simultaneously during REVLIMID therapy, during dose interruption and for 4 weeks after she has completely finished taking REVLIMID.
Highly effective forms of contraception other than tubal ligation include IUD and hormonal (birth control pills, injections, patch or implants) and a partner’s
vasectomy. Additional effective contraceptive methods include latex or synthetic condom, diaphragm and cervical cap.
Instruct patient to immediately stop taking REVLIMID and contact her healthcare provider if she becomes pregnant while taking this drug, if she misses her
menstrual period, or experiences unusual menstrual bleeding, if she stops taking birth control, or if she thinks FOR ANY REASON that she may be pregnant.
Advise patient that if her healthcare provider is not available, she should call Celgene Customer Care Center at 1-888-423-5436 [see Warnings and Precautions (5.1)
and Use in Specific Populations (8.3)].
Advise males to always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking REVLIMID and for up to 4
weeks after discontinuing REVLIMID, even if they have undergone a successful vasectomy.
Advise male patients taking REVLIMID that they must not donate sperm [see Warnings and Precautions (5.1) and Use in Specific Populations (8.3)].
All patients must be instructed to not donate blood while taking REVLIMID, during dose interruptions and for 4 weeks following discontinuation of REVLIMID [see
Warnings and Precautions (5.1)].
REVLIMID REMS program
Because of the risk of embryo-fetal toxicity, REVLIMID is only available through a restricted program called the REVLIMID REMS program [see Warnings and
Precautions (5.2)].
Patients must sign a Patient-Physician agreement form and comply with the requirements to receive REVLIMID. In particular, females of reproductive potential
must comply with the pregnancy testing, contraception requirements and participate in monthly telephone surveys. Males must comply with the contraception
requirements [see Use in Specific Populations (8.3)].
REVLIMID is available only from pharmacies that are certified in REVLIMID REMS program. Provide patients with the telephone number and website for
information on how to obtain the product.
Pregnancy Exposure Registry
Inform females there is a Pregnancy Exposure Registry that monitors pregnancy outcomes in females exposed to REVLIMID during pregnancy and that they can
contact the Pregnancy Exposure Registry by calling 1-888-423-5436 [see Use in Specific Populations (8.1)].
Hematologic Toxicity
Inform patients that REVLIMID is associated with significant neutropenia and thrombocytopenia [see Boxed Warning and Warnings and Precautions (5.3)].
Venous and Arterial Thromboembolism
Inform patients of the risk of thrombosis including DVT, PE, MI, and stroke and to report immediately any signs and symptoms suggestive of these events for
evaluation [see Boxed Warning and Warnings and Precautions (5.4)].
Increased Mortality in Patients with CLL
Inform patients that REVLIMID had increased mortality in patients with CLL and serious adverse cardiovascular reactions, including atrial fibrillation, myocardial
infarction, and cardiac failure [see Warnings and Precautions (5.5)].
Second Primary Malignancies
Inform patients of the potential risk of developing second primary malignancies during treatment with REVLIMID [see Warnings and Precautions (5.6)].
Hepatotoxicity
Inform patients of the risk of hepatotoxicity, including hepatic failure and death, and to report any signs and symptoms associated with this event to their healthcare
provider for evaluation [see Warnings and Precautions (5.8)].
Severe Cutaneous Reactions Including Hypersensitivity Reactions
Inform patients of the potential for severe reactions including hypersensitivity, angioedema, Stevens-Johnson Syndrome, toxic epidermal necrolysis or drug reaction
with eosinophilia and systemic symptoms if they had such a reaction to thalidomide and report symptoms associated with these events to their healthcare provider for
evaluation [see Warnings and Precautions (5.9)].
Tumor Lysis Syndrome
Inform patients of the potential risk of tumor lysis syndrome and to report any signs and symptoms associated with this event to their healthcare provider for evaluation
[see Warnings and Precautions (5.10)].
Tumor Flare Reaction
Inform patients of the potential risk of tumor flare reaction and to report any signs and symptoms associated with this event to their healthcare provider for evaluation
[see Warnings and Precautions (5.11)].
Early Mortality in Patients with MCL
Inform patients with MCL of the potential for early death [see Warnings and Precautions (5.14)].
Reference ID: 4439576
47
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Dosing Instructions
Inform patients how to take REVLIMID [see Dosage and Administration (2)]
REVLIMID should be taken once daily at about the same time each day,
REVLIMID may be taken either with or without food.
The capsules should not be opened, broken, or chewed. REVLIMID should be swallowed whole with water.
Instruct patients that if they miss a dose of REVLIMID, they may still take it up to 12 hours after the time they would normally take it. If more than 12 hours
have elapsed, they should be instructed to skip the dose for that day. The next day, they should take REVLIMID at the usual time. Warn patients to not take
2 doses to make up for the one that they missed.
Manufactured for: Celgene Corporation
86 Morris Avenue
Summit, NJ 07901
REVLIMID
®
and REVLIMID REMS
®
are registered trademarks of Celgene Corporation.
Pat. www.celgene.com/therapies
© 2005-2019 Celgene Corporation, All Rights Reserved.
RevPlyPI.026/MG.026
Reference ID: 4439576
48
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

MEDICATION GUIDE
REVLIMID
®
(rev-li-mid)
(lenalidomide)
capsules
What is the most important information I should know about REVLIMID?
Before you begin taking REVLIMID, you must read and agree to all of the instructions in the REVLIMID
REMS
®
program. Before prescribing REVLIMID, your healthcare provider will explain the REVLIMID
REMS program to you and have you sign the Patient-Physician Agreement Form.
REVLIMID may cause serious side effects including:
• Possible birth defects (deformed babies) or death of an unborn baby. Females who are
pregnant or who plan to become pregnant must not take REVLIMID.
REVLIMID is similar to the medicine thalidomide. We know thalidomide can cause severe life-
threatening birth defects. REVLIMID has not been tested in pregnant females. REVLIMID has
harmed unborn animals in animal testing.
Females must not get pregnant:
o For at least 4 weeks before starting REVLIMID
o While taking REVLIMID
o During any breaks (interruptions) in your treatment with REVLIMID
o For at least 4 weeks after stopping REVLIMID
Females who can become pregnant:
o Will have pregnancy tests weekly for 4 weeks, then every 4 weeks if your menstrual cycle is
regular, or every 2 weeks if your menstrual cycle is irregular.
o If you miss your period or have unusual bleeding, you will need to have a pregnancy test and
receive counseling.
o Must agree to use two acceptable forms of birth control at the same time, for at least 4 weeks
before, while taking, during any breaks (interruptions) in your treatment, and for at least 4
weeks after stopping REVLIMID.
o Talk with your healthcare provider to find out about options for acceptable forms of birth control
that you may use to prevent pregnancy before, during, and after treatment with REVLIMID.
o If you had unprotected sex or if you think your birth control has failed, stop taking REVLIMID
immediately and call your healthcare provider right away.
If you become pregnant while taking REVLIMID, stop taking it right away and call your
healthcare provider. If your healthcare provider is not available, you can call Celgene Customer
Care Center at 1-888-423-5436. Healthcare providers and patients should report all cases of
pregnancy to:
o FDA MedWatch at 1-800-FDA-1088, and
o Celgene Corporation at 1-888-423-5436
There is a pregnancy exposure registry that monitors the outcomes of females who take REVLIMID
during pregnancy, or if their male partner takes REVLIMID and they are exposed during
pregnancy. You can enroll in this registry by calling Celgene Corporation at the phone number
listed above.
REVLIMID can pass into human semen:
o Males, including those who have had a vasectomy, must always use a latex or synthetic
condom during any sexual contact with a pregnant female or a female that can become
pregnant while taking REVLIMID, during any breaks (interruptions) in your treatment with
REVLIMID, and for up to 4 weeks after stopping REVLIMID.
o Do not have unprotected sexual contact with a female who is or could become pregnant. Tell
your healthcare provider if you do have unprotected sexual contact with a female who is or
could become pregnant.
o Do not donate sperm while taking REVLIMID, during any breaks (interruptions) in your
treatment, and for 4 weeks after stopping REVLIMID. If a female becomes pregnant with your
sperm, the baby may be exposed to REVLIMID and may be born with birth defects.
Men, if your female partner becomes pregnant, you should call your healthcare provider
ri
g
ht awa
y
.
Reference ID: 4439576
49
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

• Low white blood cells (neutropenia) and low platelets (thrombocytopenia). REVLIMID causes
low white blood cells and low platelets in most people. You may need a blood transfusion or certain
medicines if your blood counts drop too low. Your healthcare provider should check your blood
counts often especially during the first several months of treatment with REVLIMID, and then at
least monthly. Tell your healthcare provider if you develop any bleeding or bruising, during
treatment with REVLIMID.
• Blood clots. Blood clots in the arteries, veins, and lungs happen more often in people who take
REVLIMID. This risk is even higher for people with multiple myeloma who take the medicine
dexamethasone with REVLIMID. Heart attacks and strokes also happen more often in people who
take REVLIMID with dexamethasone. To reduce this increased risk, most people who take
REVLIMID will also take a blood thinner medicine.
Before taking REVLIMID, tell your healthcare provider:
o If you have had a blood clot in the past
o If you have high blood pressure, smoke, or if you have been told you have a high level of fat in
your blood (hyperlipidemia)
o About all the medicines you take. Certain other medicines can also increase your risk for
blood clots
Call your healthcare provider or get medical help right away if you get any of the following
during treatment with REVLIMID:
Signs or symptoms of a blood clot in the lung, arm, or leg may include: shortness of
breath, chest pain, or arm or leg swelling
Signs or symptoms of a heart attack may include: chest pain that may spread to the
arms, neck, jaw, back, or stomach area (abdomen), feeling sweaty, shortness of breath,
feeling sick or vomiting
Signs or symptoms of stroke may include: sudden numbness or weakness, especially
on one side of the body, severe headache or confusion, or problems with vision, speech,
or balance
What is REVLIMID?
REVLIMID is a prescription medicine, used to treat adults with:
• multiple myeloma (MM)
o in combination with the medicine dexamethasone, or
o as maintenance treatment after autologous hematopoietic stem cell transplantation (a type of
stem cell transplant that uses your own stem cells)
• a condition called myelodysplastic syndromes (MDS). REVLIMID is for the type of MDS with a
chromosome problem where part of chromosome 5 is missing. This type of MDS is known as
deletion 5q MDS. People with this type of MDS may have low red blood cell counts that require
treatment with blood transfusions.
• mantle cell lymphoma (MCL) when the disease comes back or becomes worse after treatment with
2 prior medicines, one of which included bortezomib. MCL is a cancer of a type of white blood cell
called lymphocytes that are in the lymph nodes.
• follicular lymphoma (FL) or marginal zone lymphoma (MZL)
o in combination with a rituximab product, and
o who have previously been treated for their FL or MZL
FL and MZL are types of cancer of white blood cells called B-cell lymphocytes that are found in the
lymph nodes and spleen.
REVLIMID should not be used to treat people who have chronic lymphocytic leukemia (CLL) unless
they are participants in a controlled clinical trial.
It is not known if REVLIMID is safe and effective in children.
Who should not take REVLIMID?
Do not take REVLIMID if you:
• are pregnant, plan to become pregnant, or become pregnant during treatment with
REVLIMID. See “What is the most important information I should know about REVLIMID?”
• are allergic to lenalidomide or any of the ingredients in REVLIMID. See the end of this Medication
Guide for a complete list of in
g
redients in REVLIMID.
Reference ID: 4439576
50
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

What should I tell my healthcare provider before taking REVLIMID?
Before you take REVLIMID, tell your healthcare provider about all of your medical conditions,
including if you:
• have liver problems
• have kidney problems or receive kidney dialysis treatment
• have thyroid problems
• have had a serious skin rash with thalidomide treatment. You should not take REVLIMID.
• are lactose intolerant. REVLIMID contains lactose.
• are breastfeeding. Do not breastfeed during treatment with REVLIMID. It is not known if
REVLIMID passes into your breast milk and can harm your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-
counter medicines, vitamins, and herbal supplements. REVLIMID and other medicines may affect each
other, causing serious side effects. Talk with your healthcare provider before taking any new
medicines.
Know the medicines
y
ou take. Keep a list of them to show
y
our healthcare provider and pharmacist.
How should I take REVLIMID?
• Take REVLIMID exactly as prescribed and follow all the instructions of the REVLIMID REMS
program
• Swallow REVLIMID capsules whole with water 1 time a day. Do not open, break, or chew your
capsules.
• REVLIMID may be taken with or without food.
• Take REVLIMID at about the same time each day.
• Do not open or break REVLIMID capsules or handle them any more than needed.
o If powder from the REVLIMID capsule comes in contact with your skin, wash the skin right
away with soap and water.
o If powder from the REVLIMID capsule comes in contact with the inside of your eyes, nose, or
mouth, flush well with water.
• If you miss a dose of REVLIMID and it has been less than 12 hours since your regular time, take it
as soon as you remember. If it has been more than 12 hours, just skip your missed dose. Do not
take 2 doses at the same time.
• If
y
ou take too much REVLIMID, call
y
our healthcare provider ri
g
ht awa
y
.
What should I avoid while taking REVLIMID?
• See “What is the most important information I should know about REVLIMID?”
• Females: Do not get pregnant and do not breastfeed while taking REVLIMID.
• Males: Do not donate sperm.
• Do not share REVLIMID with other people. It may cause birth defects and other serious
problems.
• Do not donate blood while you take REVLIMID, during any breaks (interruptions) in your
treatment, and for 4 weeks after stopping REVLIMID. If someone who is pregnant gets your
donated blood, her bab
y
ma
y
be exposed to REVLIMID and ma
y
be born with birth defects.
What are the possible side effects of REVLIMID?
REVLIMID can cause serious side effects, including:
• See “What is the most important information I should know about REVLIMID?”
• Increased risk of death in people who have chronic lymphocytic leukemia (CLL). People with
CLL who take REVLIMID have an increased risk of death compared with people who take the
medicine chlorambucil. REVLIMID may cause you to have serious heart problems that can lead to
death, including atrial fibrillation, heart attack, or heart failure. You should not take REVLIMID if you
have CLL unless you are participating in a controlled clinical trial.
• Risk of new cancers (malignancies). An increase in new (second) cancers has happened in
patients who received REVLIMID and melphalan, or a blood stem cell transplant, including certain
blood cancers, such as acute myelogenous leukemia (AML), and myelodysplastic syndrome (MDS)
and certain other types of cancers of the skin and other organs. Talk with your healthcare provider
about your risk of developing new cancers if you take REVLIMID. Your healthcare provider will
check
y
ou for new cancers durin
g y
our treatment with REVLIMID.
Reference ID: 4439576
51
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

• Severe liver problems, including liver failure and death. Your healthcare provider should do
blood tests to check your liver function during your treatment with REVLIMID. Tell your healthcare
provider right away if you develop any of the following symptoms of liver problems:
o yellowing of your skin or the white part of
your eyes (jaundice)
o dark or brown (tea-colored) urine
o pain on the upper right side of your stomach
area (abdomen)
o bleeding or bruising more easily than
normal
o
feelin
g
ver
y
tired
• Severe skin reactions including severe allergic reactions can happen with REVLIMID and may
cause death. Call your healthcare provider right away if you develop any of these signs or
symptoms of a severe allergic reaction or severe skin reaction during treatment with REVLIMID:
o swelling of your face, eyes, lips, tongue,
throat
o trouble swallowing
o trouble breathin
g
o skin rash, hives, or peeling of your skin
o blisters
o rash with fever and or swollen glands
• Tumor lysis syndrome (TLS). TLS is caused by the fast breakdown of cancer cells. TLS can
cause kidney failure and the need for dialysis treatment, abnormal heart rhythm, seizure and
sometimes death. Your healthcare provider may do blood tests to check you for TLS.
• Worsening of your tumor (tumor flare reaction). Tell your healthcare provider if you get any of
these symptoms of tumor flare reaction while taking REVLIMID: tender swollen lymph nodes, low
grade fever, pain, or rash.
Your healthcare provider may tell you to decrease your dose, temporarily stop or permanently stop
taking REVLIMID if you develop certain serious side effects during treatment with REVLIMID.
• Thyroid problems. Your healthcare provider may check your thyroid function before you start
taking REVLIMID and during treatment with REVLIMID.
• Risk of Early Death in MCL. In people who have Mantle Cell Lymphoma (MCL), there may be a
risk of dying sooner (early death) when taking REVLIMID. Talk with your healthcare provider about
any concerns and possible risk factors.
The most common side effects of REVLIMID include:
diarrhea
rash
nausea
constipation
tiredness or weakness
fever
itching
swelling of your arms,
hands, legs, feet and skin
sleep problems (insomnia)
headache
muscle cramps or spasms
shortness of breath
cough, sore throat, and
other symptoms of a cold
upper respiratory tract infection or
bronchitis
inflammation of the stomach and
intestine (“stomach flu”)
nose bleed
shaking or trembling (tremor)
joint aches
pain in your back or stomach-area
(
abdomen
)
These are not all the possible side effects of REVLIMID.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-
FD
A
-1088.
How should I store REVLIMID?
• Store REVLIMID at room temperature between 68°F to 77°F (20°C to 25°C).
• Return any unused REVLIMID to Celgene or your healthcare provider.
Keep REVLIMID and all medicines out of the reach of children.
General information about the safe and effective use of REVLIMID.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do
not take REVLIMID for conditions for which it was not prescribed. Do not give REVLIMID to other
people, even if they have the same symptoms you have. It may harm them and may cause birth
defects.
If you would like more information, talk with your healthcare provider. You can ask your healthcare
provider or pharmacist for information about REVLIMID that is written for health professionals.
What are the in
g
redients in REVLIMID?
Reference ID: 4439576
52
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Active ingredient: lenalidomide
Inactive ingredients: lactose anhydrous, microcrystalline cellulose, croscarmellose sodium, and
magnesium stearate.
The 5 mg and 25 mg capsule shell contains gelatin, titanium dioxide and black ink.
The 2.5 and 10 mg capsule shell contains gelatin, FD&C blue #2, yellow iron oxide, titanium dioxide
and black ink.
The 15 mg capsule shell contains gelatin, FD&C blue #2, titanium dioxide and black ink.
The 20 mg capsule shell contains gelatin, FD&C blue #2, yellow iron oxide, titanium dioxide and black
ink.
Manufactured for: Celgene Corporation, 86 Morris Avenue, Summit, NJ 07901
REVLIMID
®
and REVLIMID REMS
®
are registered trademarks of Celgene Corporation.
Pat. http://www.celgene.com/therapies © 2005-2019 Celgene Corporation All rights reserved. REVPlyMG.026 5/2019
For more information, call 1-888-423-5436 or go to www.CelgeneRiskManagement.com.
This Medication Guide has been approved by the U.S. Food and Drug Administration
Revised: May 2019
Reference ID: 4439576
53
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
