HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use
DAURISMO safely and effectively. See full prescribing information for
DAURISMO.
DAURISMO
TM
(glasdegib) tablets, for oral use
Initial U.S. Approval: 2018
WARNING: EMBRYO-FETAL TOXICITY
See full prescribing information for complete boxed warning.
DAURISMO can cause embryo-fetal death or severe birth defects
when administered to a pregnant woman. DAURISMO is
embryotoxic, fetotoxic, and teratogenic in animals. (5.1, 8.1)
Conduct pregnancy testing in females of reproductive potential
prior to initiation of DAURISMO treatment. Advise females of
reproductive potential to use effective contraception during
treatment with DAURISMO and for at least 30 days after the last
dose. (5.1, 8.1, 8.3)
Advise males of the potential risk of exposure through semen and to
use condoms with a pregnant partner or a female partner of
reproductive potential during treatment with DAURISMO and for
at least 30 days after the last dose to avoid potential drug exposure.
(5.1, 8.3)
--------------------------- INDICATIONS AND U SAGE ----------------------------
DAURISMO is a hedgehog pathway inhibitor indicated, in combination with
low-dose cytarabine, for the treatment of newly-diagnosed acute myeloid
leukemia (AML) in adult patients who are ≥75 years old or who have
comorbidities that preclude use of intensive induction chemotherapy. (1)
Limitation of Use: DAURISMO has not been studied in patients with the
comorbidities of severe renal impairment or moderate-to-severe hepatic
impairment.
----------------------- DOSAGE AND ADMINISTRATION -----------------------
Recommended dose: 100 mg orally, once daily. (2.1)
--------------------- DOSAGE FORMS AND STRENGTHS ---------------------
Tablets: 100 mg, 25 mg. (3)
------------------------------ CONTRAINDICATIONS ------------------------------
None. (4)
----------------------- WARNINGS AND PRECAUTIONS -----------------------
Blood Donation: Advise patients not to donate blood or blood products
during treatment with DAURISMO and for at least 30 days after the last
dose. (5.1)
QTc Interval Prolongation: Monitor electrocardiograms and electrolytes. If
QTc prolongation occurs, interrupt treatment with DAURISMO. (2.2, 5.2)
------------------------------ ADVERSE REACTIONS ------------------------------
Most common adverse reactions (incidence ≥20%) are anemia, fatigue,
hemorrhage, febrile neutropenia, musculoskeletal pain, nausea, edema,
thrombocytopenia, dyspnea, decreased appetite, dysgeusia, mucositis,
constipation, and rash. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at
1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
------------------------------DRUG INTERACTIONS-------------------------------
Strong CYP3A4 Inhibitors: Consider alternative therapies that are not
strong CYP3A inhibitors or monitor for increased risk of adverse reactions,
including QTc interval prolongation. (7)
Strong CYP3A4 Inducers: Avoid concomitant use with DAURISMO. (7)
QTc Prolonging Drugs: Avoid co-administration with DAURISMO. If
co-administration is unavoidable, monitor for increased risk of QTc
interval prolongation. (7)
----------------------- USE IN SPECIFIC POPULATIONS -----------------------
Lactation: Advise women not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication
Guide.
Revised: 11/2018
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: EMBRYO-FETAL TOXICITY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose and Schedule
2.2 Monitoring and Dose Modifications
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
5.2 QT
c
Interval Prolongation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
* Sections or subsections omitted from the full prescribing information are
not listed.
1
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

FULL PRESCRIBING INFORMATION
WARNING: EMBRYO-FETAL TOXICITY
DAURISMO can cause embryo-fetal death or severe birth defects when administered to a
pregnant woman. DAURISMO is embryotoxic, fetotoxic, and teratogenic in animals [see
Warnings and Precautions (5.1), Use in Specific Populations (8.1)].
Conduct pregnancy testing in females of reproductive potential prior to initiation of
DAURISMO treatment. Advise females of reproductive potential to use effective
contraception during treatment with DAURISMO and for at least 30 days after the last dose
[see Warnings and Precautions (5.1), Use in Specific Populations (8.1, 8.3)].
Advise males of the potential risk of DAURISMO exposure through semen and to use
condoms with a pregnant partner or a female partner of reproductive potential during
treatment with DAURISMO and for at least 30 days after the last dose to avoid potential
drug exposure [see Warnings and Precautions (5.1), Use in Specific Populations (8.3)].
1 INDICATIONS AND USAGE
DAURISMO is indicated, in combination with low-dose cytarabine, for the treatment of newly-diagnosed acute
myeloid leukemia (AML) in adult patients who are >75 years old or who have comorbidities that preclude use
of intensive induction chemotherapy.
Limitation of Use: DAURISMO has not been studied in patients with the comorbidities of severe renal
impairment or moderate-to-severe hepatic impairment.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose and Schedule
The recommended dose of DAURISMO is 100 mg orally once daily on days 1 to 28 in combination with
cytarabine 20 mg subcutaneously twice daily on days 1 to 10 of each 28-day cycle in the absence of
unacceptable toxicity or loss of disease control. For patients without unacceptable toxicity, treat for a minimum
of 6 cycles to allow time for clinical response.
Administer DAURISMO with or without food. Do not split or crush DAURISMO tablets. Administer
DAURISMO about the same time each day. If a dose of DAURISMO is vomited, do not administer a
replacement dose; wait until the next scheduled dose is due. If a dose of DAURISMO is missed or not taken
at the usual time, administer the dose as soon as possible and at least 12 hours prior to the next scheduled dose.
Return to the normal schedule the following day. Do not administer 2 doses of DAURISMO within 12 hours.
2.2 Monitoring and Dose Modifications
Assess complete blood counts, electrolytes, renal, and hepatic function prior to the initiation of DAURISMO
and at least once weekly for the first month. Monitor electrolytes and renal function once monthly for the
2
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
duration of therapy. Obtain serum creatine kinase levels prior to initiating DAURISMO and as indicated
clinically thereafter (e.g., if muscle symptoms are reported). Monitor electrocardiograms (ECGs) prior to the
initiation of DAURISMO, approximately one week after initiation, and then once monthly for the next two
months to assess for QTc prolongation. Repeat ECG if abnormal. Certain patients may require more frequent
and ongoing ECG monitoring [see Warnings and Precautions (5.2)]. Manage any abnormalities promptly [see
Adverse Reactions (6.1)].
See Table 1 for dose modification guidelines for patients who develop an adverse reaction.
Table 1. Recommended Dose Modifications for Adverse Reactions
Adverse Reaction Recommended Action
QTc interval
prolongation on at
least 2 separate
electrocardiograms
(ECGs)
QTc interval greater
than 480 ms to
500 ms
Assess electrolyte levels and supplement as clinically indicated.
Review and adjust concomitant medications with known QTc
interval-prolonging effects [see Drug Interactions (7)].
Monitor ECGs at least weekly for 2 weeks following resolution of
QTc prolongation to less than or equal to 480 ms.
QTc interval greater Assess electrolyte levels and supplement as clinically indicated.
than 500 ms
Review and adjust concomitant medications with known QTc
interval-prolonging effects [see Drug Interactions (7)].
Interrupt DAURISMO.
Resume DAURISMO at a reduced dose of 50 mg once daily
when QTc interval returns to within 30 ms of baseline or less than
or equal to 480 ms.
Monitor ECGs at least weekly for 2 weeks following resolution of
QTc prolongation.
Consider re-escalating the dose of DAURISMO to 100 mg daily
if an alternative etiology for the QTc prolongation can be
identified.
QTc interval
prolongation with
life-threatening
arrhythmia
Discontinue DAURISMO permanently.
Hematologic
toxicity
Platelets less than
10 Gi/L for more
than 42 da
y
s in the
Discontinue DAURISMO and low-dose cytarabine permanently.
3
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Table 1. Recommended Dose Modifications for Adverse Reactions
Adverse Reaction Recommended Action
absence of disease
Neutrophil count
less than 0.5 Gi/L
for more than
42 days in the
absence of disease
Discontinue DAURISMO and low-dose cytarabine permanently.
Nonhematologic
toxicity
Grade 3
*
Interrupt DAURISMO and/or low-dose cytarabine until
symptoms reduce to mild or return to baseline.
Resume DAURISMO at the same dose level, or at a reduced dose
of 50 mg.
Resume low-dose cytarabine at the same dose level, or at a
reduced dose of 15 mg or 10 mg.
If toxicity recurs, discontinue DAURISMO and low-dose
cytarabine.
If toxicity is attributable to DAURISMO only, low-dose
cytarabine may be continued.
Grade 4
*
Discontinue DAURISMO and low-dose cytarabine permanently.
*Grade 1 is mild, Grade 2 is moderate, Grade 3 is severe, Grade 4 is life-threatening.
3 DOSAGE FORMS AND STRENGTHS
DAURISMO 100 mg tablets: round, pale orange film-coated tablet debossed with “Pfizer” on one side and
“GLS 100” on the other.
DAURISMO 25 mg tablets: round, yellow film-coated tablet debossed with “Pfizer” on one side and “GLS 25”
on the other.
4 CONTRAINDICATIONS
None.
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal embryo-fetal developmental toxicity studies,
DAURISMO can cause embryo-fetal death or severe birth defects when administered to a pregnant woman.
4
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

There are no clinical data on the use of DAURISMO in pregnant women. In animal embryo-fetal developmental
toxicity studies, glasdegib caused embryotoxicity, fetotoxicity and teratogenicity at maternal exposures that
were less than the human exposure at the recommended human dose of 100 mg [see Use in Specific Populations
(8.1, 8.2), Clinical Pharmacology (12.1)]. Advise pregnant women of the potential risk to the fetus.
Females of Reproductive Potential
DAURISMO is not recommended for use during pregnancy. Conduct pregnancy testing in female patients of
reproductive potential prior to initiating DAURISMO treatment. Advise females of reproductive potential to use
effective contraception during treatment with DAURISMO and for at least 30 days after the last dose. Advise
women not to breastfeed during treatment with DAURISMO and for at least 30 days after the last dose [see Use
in Specific Populations (8.2, 8.3)].
Males
Advise male patients with female partners of the potential risk of exposure through semen and to use effective
contraception, including a condom, even after vasectomy, to avoid drug exposure to a pregnant partner or a
female partner of reproductive potential during treatment with DAURISMO and for at least 30 days after the
last dose [see Use in Specific Populations (8.3)].
Blood Donation
Advise patients not to donate blood or blood products while taking DAURISMO and for at least 30 days after
the last dose of DAURISMO because their blood or blood products might be given to a female of reproductive
potential.
5.2 QTc Interval Prolongation
Patients treated with DAURISMO can develop QTc prolongation and ventricular arrhythmias, including
ventricular fibrillation and ventricular tachycardia. Of the 98 evaluable patients treated with DAURISMO
100 mg in combination with low-dose cytarabine in the clinical trial, 5% were found to have a QTc interval
greater than 500 ms and 4% of patients had an increase from baseline QTc greater than 60 ms. The clinical trial
excluded patients with baseline QTc of greater than 470 ms or with a history of long QT syndrome or
uncontrolled cardiovascular disease.
Monitor electrocardiograms (ECGs) and electrolytes [see Dosage and Administration (2.2)]. Concomitant use
of DAURISMO with drugs known to prolong the QTc interval and CYP3A4 inhibitors may increase the risk of
QTc interval prolongation [see Drug Interactions (7), Clinical Pharmacology (12.2)]. In patients with
congenital long QT syndrome, congestive heart failure, electrolyte abnormalities, or those who are taking
medications known to prolong the QTc interval, more frequent ECG monitoring is recommended.
Interrupt DAURISMO if QTc increases to greater than 500 ms. Discontinue DAURISMO permanently for
patients who develop QTc interval prolongation with signs or symptoms of life-threatening arrhythmia [see
Dosage and Administration (2.2)].
6 ADVERSE REACTIONS
The following clinically significant adverse reaction is described elsewhere in the labeling:
QT Interval Prolongation [see Warnings and Precautions (5.2)]
5
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the
clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not
reflect the rates observed in practice.
The safety profile of DAURISMO is based on experience in the BRIGHT AML 1003 study for 111 adults with
newly-diagnosed AML and 14 adults with other conditions for which DAURISMO is not indicated [see
Clinical Studies (14)]. Patients were treated with DAURISMO 100 mg daily in combination with low-dose
cytarabine (N=84) or low-dose cytarabine alone (N=41). The median duration of treatment in the DAURISMO
with low-dose cytarabine arm was 83 days (range 3 to 972 days), and the median duration of treatment in the
low-dose cytarabine alone arm was 47 days (range 6 to 239 days). The median exposure to DAURISMO in the
DAURISMO with low-dose cytarabine arm was 76 days (range 3 to 954 days). Thirty-two patients (38%) were
treated with DAURISMO with low-dose cytarabine for at least 6 months and 14 patients (17%) were treated for
at least 1 year.
Serious adverse reactions were reported in 79% of patients treated in the DAURISMO with low-dose cytarabine
arm. The most common (≥5%) serious adverse reactions in patients receiving DAURISMO with low-dose
cytarabine were febrile neutropenia (29%), pneumonia (23%), hemorrhage (12%), anemia (7%), and
sepsis (7%).
Dose reductions associated with adverse reactions were reported in 26% of patients treated with DAURISMO
with low-dose cytarabine, and the most common reasons (≥2%) for dose reductions due to adverse reactions
were muscle spasms (5%), fatigue (4%), febrile neutropenia (4%), anemia (2%), thrombocytopenia (2%), and
ECG QT prolonged (2%). Adverse reactions leading to permanent discontinuation were reported in 36% of
patients treated with DAURISMO with low-dose cytarabine, and the most common (≥2%) reasons for
permanent discontinuation were pneumonia (6%), febrile neutropenia (4%), sepsis (4%), sudden death (2%),
myocardial infarction (2%), nausea (2%), and renal insufficiency (2%).
Adverse reactions reported in the first 90 days of therapy on the BRIGHT AML 1003 study are shown in
Table 2.
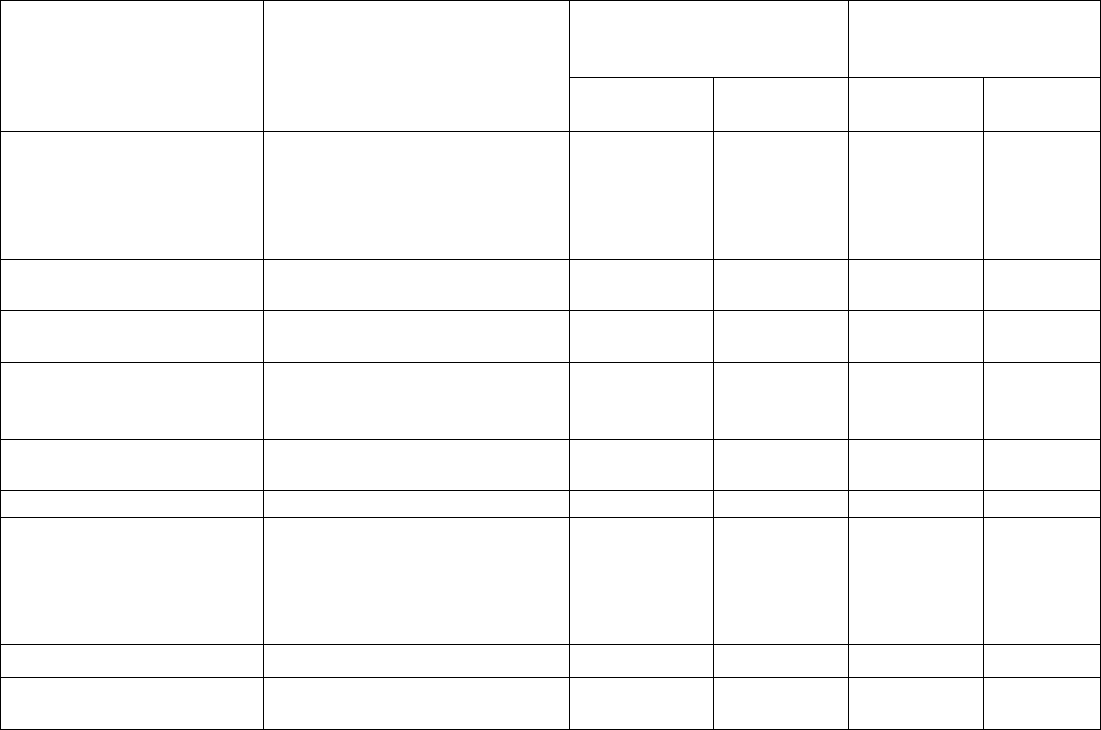
Table 2. Adverse Reactions Occurring in ≥ 10% of Patients
a,b
Within the First 90 Days of Therapy in
BRIGHT AML 1003
Body System Adverse Reactions DAURISMO With
Low-Dose Cytarabine
N=84
Low-Dose Cytarabine
N=41
All Grades
%
Grade ≥ 3
%
All Grades
%
Grade ≥ 3
%
Blood and lymphatic Anemia 43 41 42 37
system disorder Hemorrhage
c
36 6 42 12
Febrile neutropenia 31 31 22 22
Thrombocytopenia 30 30 27 24
General disorders and
Fatigue
d
36 14 32 7
administration site Edema
e
30 0 20 2
conditions Mucositis
f
21 1 12 0
Pyrexia 18 1 22 2
Chest pain
g
12 1 2 0
Musculoskeletal and
connective tissue disorders
Musculoskeletal pain
h
Muscle spas
m
i
30
15
2
0
17
5
2
0
6
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
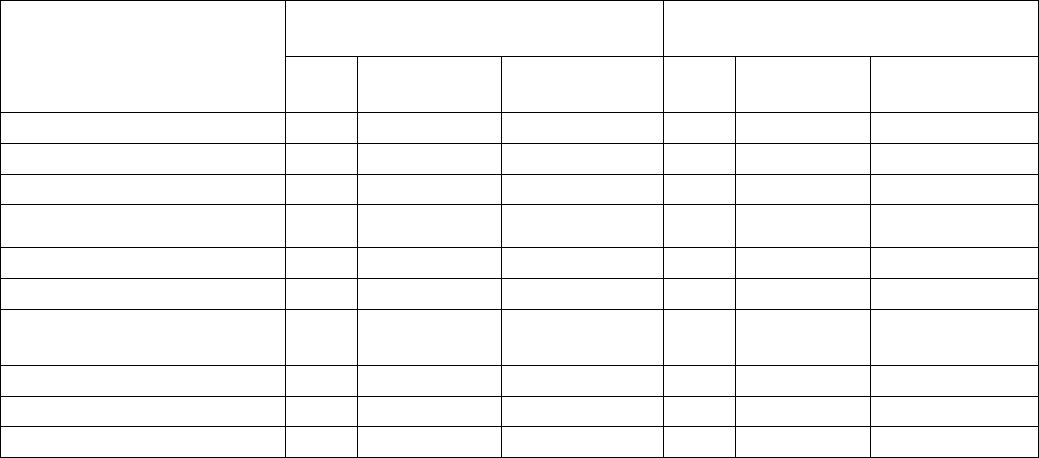
Body System Adverse Reactions DAURISMO With
Low-Dose Cytarabine
N=84
Low-Dose Cytarabine
N=41
All Grades
%
Grade ≥ 3
%
All Grades
%
Grade ≥ 3
%
Gastrointestinal disorders Nausea 29 1 12 2
Constipation 20 1 12 0
Abdominal pain
j
19 0 12 0
Diarrhea
k
18 4 22 0
Vomiting 18 2 10 2
Respiratory thoracic and
mediastinal disorders
Dyspnea
l
Cough
m
23
18
11
0
24
15
7
2
Metabolism and nutrition
disorders
Decrease appetite 21 1 7 2
Nervous system disorders Dysgeusia
n
Dizziness
Headache
21
18
12
0
1
0
2
7
10
0
0
2
Skin and subcutaneous
tissue disorders
Rash
o
20 2 7 2
Infection and infestations
Pneumonia
p
19 15 24 22
Investigations Hyponatremia 11 6 0 0
Platelet count decreased 15 15 10 10
Weight decreased 13 0 2 0
White blood cell count
decreased
11 11 5 2
Cardiac disorders Atrial arrhythmia
q
13 4 7 2
Renal and urinary
disorders
Renal insufficiency
r
19 5 10 0
Abbreviations: N = number of patients.
Preferred terms were retrieved by applying the Medical Dictionary for Regulatory Activities (MedDRA) version 19.1.
BRIGHT AML 1003 used National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Adverse reactions include events that commenced within 28 days after the last treatment dose.
a.
Adverse reactions with ≥10% incidence in the DAURISMO with low-dose cytarabine arm or the low-dose cytarabine arm are included.
b.
No Grade 5 events in the DAURISMO with low-dose cytarabine or low-dose cytarabine alone arm.
c.
Hemorrhage includes petechiae, epistaxis, hematoma, contusion, rectal hemorrhage, anal hemorrhage, ecchymosis, gingival bleeding, hematuria,
mouth hemorrhage, purpura, cerebral hemorrhage, eye contusion, eye hemorrhage, gastric hemorrhage, gastrointestinal hemorrhage, hematemesis,
hemoptysis, hemorrhage, implant site hematoma, injection site bruising, retroperitoneal hematoma, thrombotic thrombocytopenic purpura, tracheal
hemorrhage, conjunctival hemorrhage, disseminated intravascular coagulation, eyelid hematoma, hematochezia, hemorrhage intracranial,
hemorrhoidal hemorrhage, lower gastrointestinal hemorrhage, retinal hemorrhage, and subdural hematoma.
d.
Fatigue includes asthenia and fatigue.
e.
Edema includes edema peripheral, edema, fluid overload, fluid retention, and swelling face.
f.
Mucositis includes mucosal inflammation, oropharyngeal pain, stomatitis, anal ulcer, gingival pain, laryngeal inflammation, esophagitis, oral pain,
aphthous ulcer, mouth ulceration, and pharyngeal inflammation.
g.
Chest pain includes chest pain and non-cardiac chest pain.
h.
Musculoskeletal pain includes pain in extremity, arthralgia, back pain, myalgia, musculoskeletal pain, musculoskeletal chest pain, neck pain, and
bone pain.
i.
Muscle spasms includes muscle spasms and muscle tightness.
j.
Abdominal pain includes abdominal pain, abdominal pain upper, and abdominal pain lower.
k.
Diarrhea includes diarrhea, colitis, and gastroenteritis.
l.
Dyspnea includes dyspnea, hypoxia, bronchospasm, and respiratory failure.
m.
Cough includes cough and productive cough.
n.
Dysgeusia includes dysgeusia and ageusia.
o.
Rash includes rash, pruritus, erythema, skin ulcer, rash maculo-papular, and rash pruritic.
p.
Pneumonia includes pneumonia, pneumonia aspiration, and lung infection.
q.
Atrial arrhythmia includes atrial fibrillation, bradycardia, tachycardia, and sinus tachycardia.
r.
Renal insufficiency includes acute kidney injury, blood creatinine increased, oliguria, and renal failure.
The adverse reactions muscle spasms (4 in 12 patients) and decreased appetite (2 in 10 patients) worsened (i.e.
progressed from Grades ≤ 2 to Grade 3 or higher) after the first 90 days of therapy in BRIGHT AML 1003.
7
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Additional clinically-significant adverse reactions occurring in < 10% of patients treated with DAURISMO and
low-dose cytarabine in BRIGHT AML 1003 include:
Dental disorders: loose tooth and toothache
Skin and subcutaneous tissue disorders: alopecia
Cardiac disorders: QT interval prolonged
Changes in selected post-baseline laboratory values that were observed in patients with newly-diagnosed AML
and other conditions for which DAURISMO is not indicated in the clinical trial are shown in Table 3.
Table 3. Selected Laboratory Abnormalities (≥ 15% )
a
Within the First 90 Days of Therapy in BRIGHT
AML 1003
Laboratory Abnormality
DAURISMO with Low-Dose
Cytarabine
Low-Dose Cytarabine
N
All Grades
%
Grade 3 or 4
*
%
N
All Grades
%
Grade 3 or 4
*
%
Creatinine increased 81 96 1 40 80 5
Hyponatremia 81 54 7 39 41 8
Hypomagnesemia 81 33 0 39 23 0
AST increased 80 28 1 40 23 0
Blood bilirubin increased 80 25 4 39 33 3
ALT increased 80 24 0 40 28 3
Alkaline phosphatase
increase
d
80 23 0 40 28 3
Hyperkalemia 81 16 1 40 8 3
CPK increased 38 16 0 17 6 0
Hypokalemia 81 15 0 40 23 0
Abbreviations: N = number of patients; AST = aspartate aminotransferase; ALT = alanine aminotransferase; CPK = creatinine
phosphokinase.
BRIGHT AML 1003 used National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
*
Grade 1 is mild, Grade 2 is moderate, Grade 3 is severe, Grade 4 is life-threatening.
a.
Maximum severity based on the number of patients with available on-study laboratory data.
The following laboratory abnormalities worsened (i.e. progressed from Grades ≤ 2 to Grade 3 or higher) after
the first 90 days of therapy in BRIGHT AML 1003:
hypophosphatemia (8 in 38 patients), creatinine increased (2 in 39 patients), and ALT increased (2 in
40 patients).
8
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

7 DRUG INTERACTIONS
Table 4. Drug Interactions with DAURISMO
Strong CYP3A Inhibitors
Clinical Impact
Co-administration of DAURISMO with strong CYP3A inhibitors
increased glasdegib plasma concentrations [see Clinical
Pharmacology (12.3)].
Increased glasdegib concentrations may increase the risk of adverse
reactions including QTc interval prolongation [see Warnings and
Precautions (5.2)].
Prevention or
Management
Consider alternative therapies that are not strong CYP3A4 inhibitors
during treatment with DAURISMO.
Monitor patients for increased risk of adverse reactions including
QTc interval prolongation [see Warnings and Precautions (5.2)].
Strong CYP3A Inducers
Clinical Impact
Co-administration of DAURISMO with strong CYP3A inducers
decreased glasdegib plasma concentrations [see Clinical Pharmacology
(12.3)].
Prevention or
Management
Avoid co-administration of DAURISMO with strong CYP3A4 inducers.
QTc Prolonging Drugs
Clinical Impact
Co-administration of DAURISMO with QTc prolonging drugs may
increase the risk of QTc interval prolongation [see Warnings and
Precautions (5.2)].
Prevention or
Management
Avoid co-administration of QTc prolonging drugs with DAURISMO
or replace with alternative therapies.
If co-administration of a QTc prolonging drug is unavoidable, monitor
patients for increased risk of QTc interval prolongation [see Warnings
and Precautions (5.2)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action and findings in animal embryo-fetal developmental toxicity studies,
DAURISMO can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology
(12.1)]. There are no clinical data on the use of DAURISMO in pregnant women to inform of a drug-associated
risk of major birth defects and miscarriage. DAURISMO is not recommended for use during pregnancy.
Conduct pregnancy testing in female patients of reproductive potential prior to initiating treatment with
DAURISMO. Report pregnancy exposures to Pfizer at 1-800-438-1985.
In animal embryo-fetal developmental toxicity studies, repeat-dose oral administration of DAURISMO during
organogenesis at maternal exposures that were less than the human exposure at the recommended dose resulted
9
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
in embryotoxicity, fetotoxicity and teratogenicity in rats and rabbits (see Data). Advise pregnant women of the
potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population are unknown.
Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. In the
U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically
recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In embryo-fetal developmental toxicity studies, glasdegib was orally administered to pregnant rats and rabbits at
doses up to 100 mg/kg/day during the period of organogenesis. Glasdegib resulted in embryo-fetal lethality
(e.g., increased postimplantation loss and decreased numbers of live fetuses) in rats and rabbits at 50 mg/kg/day
and 5 mg/kg/day, respectively, at maternal exposures approximately 4-times and 3-times the human exposure at
the recommended dose [based on C
max
(rat) and AUC (rabbit)]. Doses of ≥ 10 mg/kg in rat [approximately
0.6-times the human exposure (C
max
) at the recommended dose] and ≥ 5 mg/kg in rabbit resulted in fetal
developmental abnormalities and malformations consisting of craniofacial malformations, malformed limbs,
paws/digits, trunk and tail, dilation of brain, malpositioned/malformed eyes, misshapen head, small tongue,
absent palate, teeth and viscera, diaphragmatic hernia, edema, heart defects, rib and vertebral abnormalities,
malformed or absent structures in the appendicular skeleton.
8.2 Lactation
Risk Summary
There are no data on the presence of glasdegib or its active metabolites in human milk, the effects of the drug on
the breastfed child, or its effect on milk production. Because of the potential for serious adverse reactions in a
breastfed child from DAURISMO, advise women who are taking DAURISMO not to breastfeed or provide
breast milk to infants or children during treatment with DAURISMO and for at least 30 days after the last dose.
8.3 Females and Males of Reproductive Potential
DAURISMO can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations
(8.1)].
Pregnancy Testing
Conduct pregnancy testing in females of reproductive potential within 7 days prior to initiating therapy with
DAURISMO.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with DAURISMO and
at least 30 days after the last dose.
Males
It is not known if glasdegib is present in semen. Advise males of the potential risk of exposure through semen
and to use effective contraception, including a condom, even after a vasectomy, to avoid drug exposure to a
pregnant partner or a female partner of reproductive potential during treatment with DAURISMO and for at
least 30 days after the last dose. Advise males to not donate semen during treatment with DAURISMO for at
least 30 days after the last dose [see Nonclinical Toxicology (13.1)].
10
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Infertility
Males
Based on findings in repeat-dose animal toxicity studies in rats, DAURISMO may impair fertility in males of
reproductive potential. Some effects on male reproductive organs did not recover [see Nonclinical Toxicology
(13.1)]. Men should seek advice on effective fertility preservation before treatment.
8.4 Pediatric Use
The safety and effectiveness of DAURISMO have not been established in pediatric patients. In repeat-dose
toxicity studies in rats, oral administration of DAURISMO resulted in adverse changes in growing bone, teeth,
and testis. Effects on bone consisted of partial to complete closure of the epiphyseal plate. Effects in growing
incisor teeth included degeneration/necrosis of ameloblasts, and complete tooth loss with oral ulceration.
Reproductive tissue toxicity was evidenced by testicular degeneration and hypospermatogenesis. These effects
in bone, teeth and testis were observed after administration of DAURISMO for 26 weeks at greater than or
equal to 50 mg/kg/day corresponding to approximately 6.6-times the steady state AUC in patients at the
recommended human dose.
8.5 Geriatric Use
Of the total number of subjects in clinical studies of DAURISMO with low-dose cytarabine (N=88), 98% of the
patients were age 65 years or older and 60% of the patients were age 75 years or older. There were insufficient
patients younger than age 65 years to determine differences in adverse reactions reported from patients older
than 65.
10 OVERDOSAGE
There is no specific antidote for DAURISMO. Management of DAURISMO overdose should include
symptomatic treatment and ECG monitoring.
Glasdegib has been administered in clinical studies up to a dose of 640 mg/day. At the highest dosage the
adverse events reported were nausea, vomiting, dehydration, fatigue and dizziness.
11 DESCRIPTION
DAURISMO (glasdegib) is a potent small molecule inhibitor of Smoothened (SMO) for oral use. It is
formulated with the maleate salt of glasdegib. The molecular formula for glasdegib maleate is C
25
H
26
N
6
O
5
. The
molecular weight for glasdegib maleate is 490.51 Daltons. The chemical name of glasdegib maleate is
1-((2R,4R)-2-(1H-benzo[d]imidazol-2-yl)-1-methylpiperidin-4-yl)-3-(4-cyanophenyl)urea maleate. The
molecular structure is shown below:
N
HN
N
H
N
Me
N
H
O
CN
CO
2
H
CO
2
H
Glasdegib maleate is a white to pale colored powder with pKa values of 1.7 and 6.1. The aqueous solubility of
glasdegib maleate is 1.7 mg/mL.
11
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

DAURISMO (glasdegib) is supplied as a film-coated tablet for oral use containing either 100 mg glasdegib
(equivalent to 131.1 mg glasdegib maleate) or 25 mg of glasdegib (equivalent to 32.8 mg glasdegib maleate)
together with microcrystalline cellulose, dibasic calcium phosphate anhydrous, sodium starch glycolate, and
magnesium stearate as inactive ingredients in the tablet. The film-coating consists of Opadry II
®
Beige
(33G170003) and Opadry II
®
Yellow (33G120011) containing: hypromellose, titanium dioxide, lactose
monohydrate, macrogol, triacetin, iron oxide yellow, and iron oxide red.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Glasdegib is an inhibitor of the Hedgehog pathway. Glasdegib binds to and inhibits Smoothened, a
transmembrane protein involved in hedgehog signal transduction.
In a murine xenotransplant model of human AML, glasdegib in combination with low-dose cytarabine,
inhibited increases in tumor size and reduced the percentage of CD45+/CD33+ blasts in the marrow to a greater
extent than glasdegib or low-dose cytarabine alone.
12.2 Pharmacodynamics
Cardiac Electrophysiology
The effect of glasdegib administration on corrected QT interval (QTc) was evaluated in a randomized,
single-dose, double-blind, 4-way crossover, placebo- and open-label moxifloxacin-controlled study in
36 healthy subjects. At therapeutic plasma concentrations for the recommended dose, achieved with a single
dose of 150 mg DAURISMO, the largest placebo and baseline-adjusted QTc interval change was 8 ms (90% CI:
6, 10 ms). At a two-fold therapeutic plasma concentration, achieved with a single dose of 300 mg DAURISMO,
the QTc change was 13 ms (90% CI: 11, 16 ms). Glasdegib is associated with concentration-dependent QTc
prolongation.
12.3 Pharmacokinetics
DAURISMO at 5 mg to 600 mg once daily (0.05 to 6 times the recommended dose) result in a dose
proportional increase in glasdegib peak concentrations (C
max
) and area under the curve over the dosing interval
(AUC
tau
). Steady-state plasma levels are reached by 8 days of daily dosing. The median accumulation ratio of
glasdegib ranged from 1.2 to 2.5 following once-daily dosing.
At DAURISMO 100 mg once daily, the geometric mean (geometric coefficient of variation, % CV) of
glasdegib C
max
was 1252 ng/mL (44%) and AUC
tau
was 17210 ng*hr/mL (54%) in patients with cancer.
Absorption
The mean absolute bioavailability of DAURISMO is 77%. Following 100 mg once daily dosing, glasdegib
median time to peak concentrations (T
max
) at steady-state ranged from 1.3 hours to 1.8 hours.
Effect of Food: A high-fat, high-calorie meal (total 800-1000 calories: 500-600 fat calories, 250 carbohydrate
calories and 150 protein calories) reduced area under the curve over time to infinity (AUC
inf
) by 16% and C
max
by 31%.
Distribution
Glasdegib is 91% bound to human plasma proteins in vitro. The geometric mean (%CV) apparent volume of
distribution (V
z
/F) was 188 L (20%) in patients with hematologic malignancies.
12
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Elimination
Glasdegib has a mean ( SD) half-life of 17.4 h (3.7) and geometric mean (%CV) apparent clearance of
6.45 L/h (25%) following 100 mg once daily dosing in patients with hematologic malignancies.
Metabolism
Glasdegib is primarily metabolized by the CYP3A4 pathway, with minor contributions by CYP2C8 and
UGT1A9. Glasdegib accounts for 69% of the total circulating drug related material in plasma.
Excretion
Following a single oral dose of 100 mg radiolabeled glasdegib, 49% (17% unchanged) of the administered dose
was eliminated in the urine and 42% (20% unchanged) of the administered dose was eliminated in the feces.
Specific Populations
Age (25 to 92 years), sex, race (White, Black, Asian), body weight (43.5 to 145.6 kg), mild hepatic impairment
(total bilirubin ≤ ULN and AST > ULN, or total bilirubin 1-1.5 x ULN and any AST) or mild to moderate renal
impairment (creatinine clearance [CLcr] 30-89 mL/min) did not have clinically meaningful effects on the
pharmacokinetics of glasdegib. The effect of moderate (total bilirubin 1.5-3 x ULN and any AST) and severe
(total bilirubin > 3 x ULN and any AST) hepatic impairment or severe renal impairment (CLcr 15-29 mL/min)
on glasdegib pharmacokinetics is unknown.
Drug Interaction Studies
Clinical Studies
Effect of Strong CYP3A4 Inhibitors on Glasdegib:
Coadminstration of ketoconazole (a strong inhibitor of CYP3A4) with DAURISMO increased the glasdegib
AUC
inf
by 2.4-fold and C
max
by 1.4-fold over glasdegib given alone [see Drug Interactions (7)].
Effect of Strong CYP3A4 Inducers on Glasdegib:
Coadminstration of rifampin (a strong inducer of CYP3A4) with DAURISMO decreased glasdegib AUC
inf
by
70% and C
max
by 35% [see Drug Interactions (7)].
Effect of Gastric Acid Reducing Agents on Glasdegib:
Coadministration of rabeprazole (a proton pump inhibitor) with DAURISMO did not alter glasdegib AUC
inf
but
decreased C
max
by 20%.
In Vitro Studies
Effect of Glasdegib on Cytochrome P450 (CYP) Substrates:
Glasdegib does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A, and does
not induce CYP1A2, CYP2B6, and CYP3A in vitro.
Effect of Transporters on Glasdegib:
Glasdegib is a substrate of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP).
Effect of Glasdegib on Transporters:
Glasdegib inhibits P-gp, BCRP, multidrug and toxin extrusion (MATE) protein 1, and MATE-2K, but not
organic anion transporting polypeptide (OATP)1B1, OATP1B3, organic anion transporter (OAT)1, OAT3, and
organic cation transporter (OCT)2 in vitro.
13
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been performed with glasdegib.
Glasdegib was not mutagenic in vitro in the bacterial reverse mutation (Ames) assay and was not clastogenic in
the in vitro chromosome aberration assay in human lymphocytes. Glasdegib was not clastogenic or aneugenic in
the rat micronucleus assay.
Based on nonclinical safety findings, glasdegib has the potential to impair reproductive function in males. Men
should seek advice on effective fertility preservation before treatment. In repeat-dose toxicity studies in rats,
findings observed in the male reproductive tract included adverse testicular changes with glasdegib at doses
50 mg/kg/day, and consisted of minimal to severe hypospermatogenesis characterized by partial to complete
loss of spermatogonia, spermatocytes and spermatids and testicular degeneration. Hypospermatogenesis did not
recover whereas testicular degeneration did recover. The dose at which testicular effects were observed in male
rats was identified as 50 mg/kg/day with corresponding systemic exposures that were approximately 6.6-times
(based on AUC) those associated with the observed human exposure at the 100 mg once daily dose.
14 CLINICAL STUDIES
The efficacy of DAURISMO in combination with low-dose cytarabine was evaluated in a multicenter, open-
label, randomized study (Study BRIGHT AML 1003, NCT01546038) that included 115 patients age 55 years
or older with newly-diagnosed AML who met at least one of the following criteria: a) age > 75 years, b) severe
cardiac disease, c) baseline Eastern Cooperative Oncology Group (ECOG) performance status of 2, or
d) baseline serum creatinine >1.3 mg/dL. Patients were randomized 2:1 to receive DAURISMO at a 100 mg
daily dose with low-dose cytarabine 20 mg subcutaneously twice daily on days 1 to 10 of a 28-day cycle
(N=77) or low-dose cytarabine alone (N=38) in 28-day cycles until disease progression or unacceptable
toxicity. Patients were stratified by cytogenetic risk (good/intermediate or poor).
The baseline demographic and disease characteristics are shown in Table 5. The two treatment arms were
generally balanced with respect to the baseline demographics and disease characteristics (see Table 5).
14
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
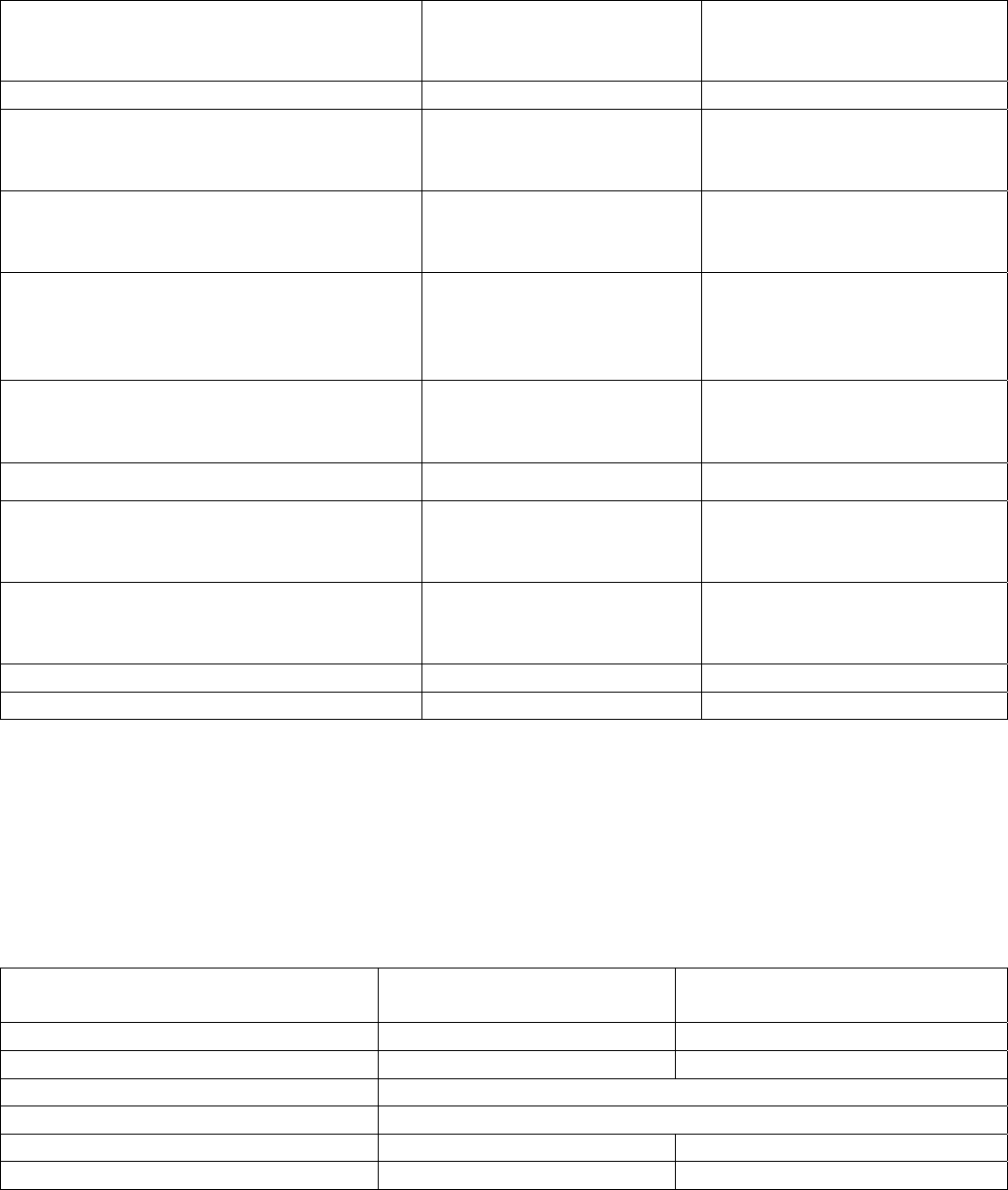
Table 5. Baseline Demographic and Disease Characteristics in Patients with AML
Demographic and Disease
Characteristics
DAURISMO With
Low-Dose Cytarabine
(N=77)
Low-Dose Cytarabine Alone
(N=38)
Demographics
Age
Median (Min, Max) (Years)
≥ 75
y
ears N (%)
77 (64, 92)
47 (61)
76 (58, 83)
23 (61)
Sex, N (%)
Male
Female
59 (77)
18 (23)
23 (61)
15 (39)
Race, N (%)
White
Black or African American
Asian
75 (97)
1 (1)
1 (1)
38 (100)
0 (0)
0 (0)
Disease History, N (%)
De Novo AML
Secondary AML
38 (49)
39 (51)
18 (47)
20 (53)
Prior Hypomethylating Agent Use 11 (14) 6 (16)
ECOG PS
a
, N (%)
0 to 1
2
35 (46)
41 (53)
20 (53)
18 (47)
Cytogenetic Risk Status, N (%)
Good/Intermediate
Poo
r
48 (62)
29 (38)
21 (55)
17 (45)
Baseline Severe Cardiac Disease 51 (66) 20 (53)
Baseline Serum Creatinine >1.3 mg/dL 15 (19) 5 (13)
Abbreviations: AML = acute myeloid leukemia; N = number of patients; ECOG PS = Eastern Cooperative Oncology Group Performance Status.
a
Baseline ECOG PS was not reported for one patient in the DAURISMO with low-dose cytarabine arm.
Efficacy was established on the basis of overall survival (OS) from the date of randomization to death from any
cause. With a median follow-up of approximately 20 months, the DAURISMO with low-dose cytarabine arm
was superior to low-dose cytarabine alone arm (Figure 1). The efficacy results are shown in Table 6.
Improvement in OS was consistent across prespecified cytogenetic risk subgroups.
Table 6. Efficacy Results From BRIGHT AML 1003
Endpoint/Study Population DAURISMO With
Low-Dose Cytarabine
Low-Dose Cytarabine Alone
OS N=77 N=38
Median survival, months (95% CI) 8.3 (4.4, 12.2) 4.3 (1.9, 5.7)
Hazard ratio (95% CI)
a
0.46 (0.30, 0.71)
p-value
b
0.0002
CR N=14 N=1
CR rate (in %, 95% CI) 18.2 (10.3, 28.6) 2.6 (0.1, 13.8)
Abbreviations: AML = acute myeloid leukemia; N = number of patients; OS = overall survival; CI = confidence interval; CR = complete response.
a.
Hazard ratio (DAURISMO with low-dose cytarabine/low-dose cytarabine alone) based on the Cox Proportional hazards model stratified by
cytogenetic risk.
b.
1-sided p-value from log-rank test stratified by cytogenetic risk.
15
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
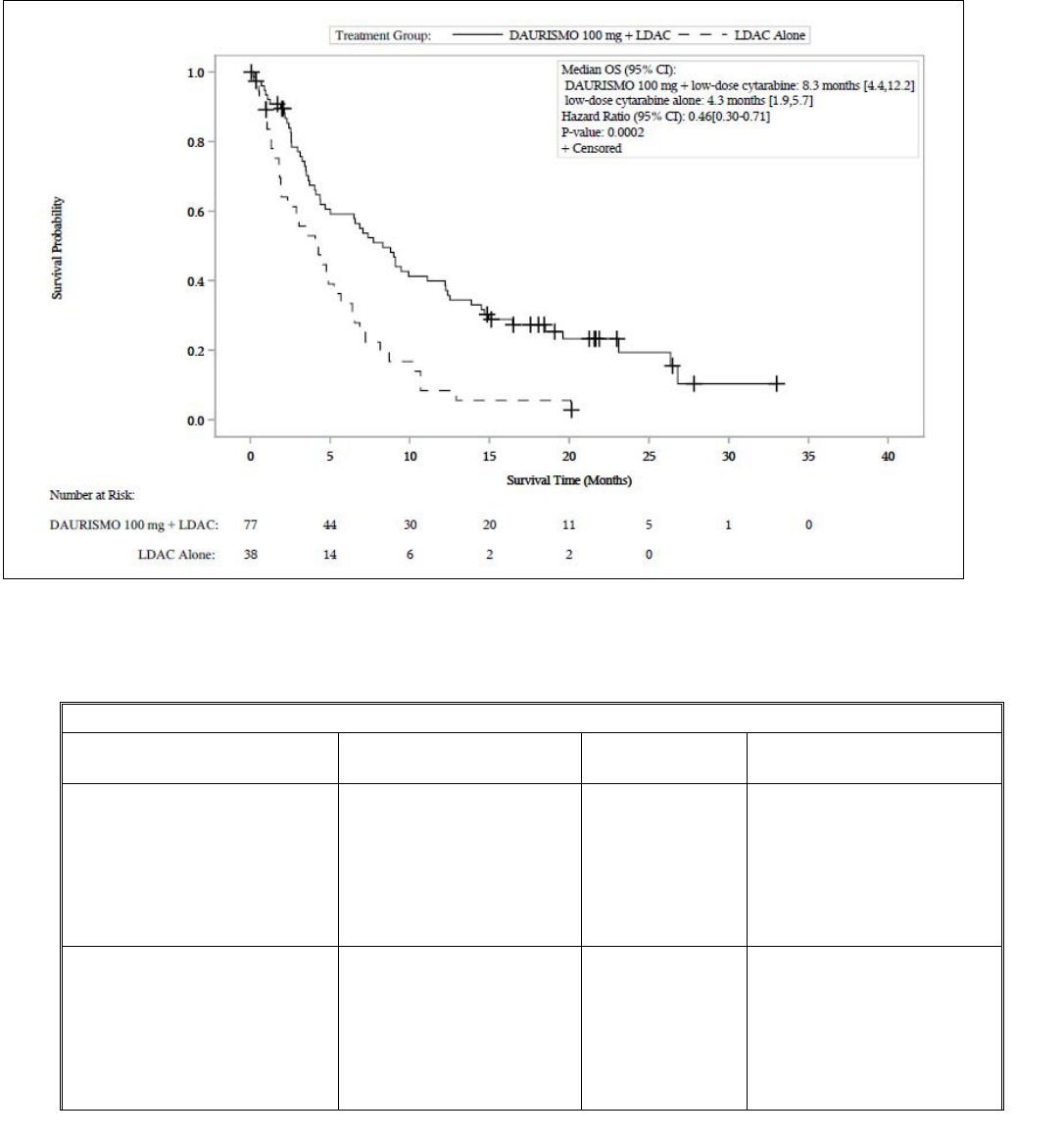
Figure 1. BRIGHT AML 1003 – Kaplan-Meier Plot of Overall Survival for Patients with AML
Abbreviations: CI = confidence interval; OS = overall survival; LDAC = low-dose cytarabine.
16 HOW SUPPLIED/STORAGE AND HANDLING
DAURISMO is supplied in the following strengths and package configurations:
DAURISMO fil
m
-coated tablets
Packa
g
e Confi
g
uratio
n
Tablet Stren
g
th (m
g
) NDC Print(description)
30 count bottle
100
m
g
0069-1531-30
100 mg strength: 11 mm
round, pale orange
film-coated tablet
debossed with “Pfizer”
on one side and “GLS
100” on the other
60 count bottle
25 m
g
0069-0298-60
25 mg strength: 7 mm
round, yellow
film-coated tablet
debossed with “Pfizer”
on one side and “GLS
25” on the other
Store at 20
o
C to 25
o
C (68
o
F to 77
o
F); excursions permitted between 15
o
C to 30
o
C (59
o
F to 86
o
F).
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Advise patients of the risks of DAURISMO treatment:
16
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Embryo-Fetal Toxicity
Advise female patients of the potential risk to a fetus and to inform their healthcare provider of a known
or suspected pregnancy. Advise female patients and female partners of male patients to contact their
healthcare provider with a known or suspected pregnancy [see Warnings and Precautions (5.1), Use in
Specific Populations (8.1, 8.3)].
Females and Males of Reproductive Potential
Advise females of reproductive potential to use effective contraception during treatment with
DAURISMO and for at least 30 days after the last dose.
Advise males of the potential risk of exposure through semen and to use effective contraception,
including a condom, even after a vasectomy, to avoid drug exposure to a pregnant partner or a female
partner of reproductive potential during treatment with DAURISMO and for at least 30 days after the
last dose [see Use in Specific Populations (8.3)].
Semen Donation
Advise males not to donate semen during treatment with DAURISMO and for at least 30 days after the
last dose of DAURISMO[see Use in Specific Populations (8.3)].
Lactation
Advise women not to breastfeed during treatment with DAURISMO and for at least 30 days after the
last dose of DAURISMO [see Use in Specific Populations (8.2)].
Blood Donation
Advise patients not to donate blood or blood products during treatment with DAURISMO and for at
least 30 days after the last dose of DAURISMO [see Warnings and Precautions (5.1)].
Infertility
Advise males of reproductive potential of the potential for impaired fertility from DAURISMO. Advise
male patients to seek advice on effective fertility preservation before treatment [see Use in Specific
Populations (8.3), Nonclinical Toxicology (13.1)].
QT Interval Prolongation
Inform patients of signs and symptoms that may be indicative of significant QT interval prolongation.
Advise patients to contact their healthcare provider immediately in the event of syncope, pre-syncopal
symptoms, and cardiac palpitations [see Warnings and Precautions (5.2)].
This product’s label may have been updated. For current full prescribing information, please visit
www.DAURISMO.com.
LAB-1284-0.6
17
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

MEDICATION GUIDE
DAURISMO
TM
(DOOR-is-moe)
(glasdegib) tablets
What is the most important information I should know about DAURISMO?
DAURISMO can cause your baby to die before it is born (be stillborn) or cause your baby to have severe birth
defects.
For females who can become pregnant:
You should talk to your healthcare provider about the risks of DAURISMO to your unborn child.
Your healthcare provider will do a pregnancy test within 7 days before you start taking DAURISMO.
You should not use DAURISMO during pregnancy.
You should use effective birth control during treatment and for at least 30 days after your last dose of DAURISMO.
Talk with your healthcare provider about what birth control method is right for you during this time.
Talk to your healthcare provider right away if you have unprotected sex or if you think that your birth control has
failed.
Tell your healthcare provider right away if you become pregnant or think that you may be pregnant.
For males:
It is not known if DAURISMO is present in semen. Do not donate semen during treatment with DAURISMO and for
at least 30 days after your last dose.
You should always use effective birth control, including a condom, even if you have had a vasectomy, during sex
with female partners who are pregnant or who are able to become pregnant, during treatment with DAURISMO
and for at least 30 days after your last dose to protect your female partner from being exposed to DAURISMO.
Tell your healthcare provider right away if your partner becomes pregnant or thinks she is pregnant while you are
taking DAURISMO.
Exposure to DAURISMO during pregnancy:
If you think that you or your female partner may have been exposed to DAURISMO during pregnancy, talk to your
healthcare provider right away. If you become pregnant during treatment with DAURISMO, you or your healthcare
provider should report your pregnancy to Pfizer at 1-800-438-1985.
What is DAURISMO?
DAURISMO is a prescription medicine that is used with the medicine cytarabine to treat newly-diagnosed acute
myeloid leukemia (AML) in adults who:
are 75 years of age or older, or
have other medical conditions that prevent the use of standard chemotherapy
DAURISMO has not been studied in people with severe kidney problems or moderate-to-severe liver problems.
It is not known if DAURISMO is safe and effective in children.
Before you take DAURISMO, tell your healthcare provider about all of your medical conditions, including if
you:
have heart problems, including a condition called long QT syndrome.
abnormal blood salt (electrolytes) levels.
are pregnant or plan to become pregnant. See “What is the most important information I should know about
DAURISMO?”
are breastfeeding or plan to breastfeed. It is not known if DAURISMO passes into your breast milk. Do not
breastfeed or provide breast milk to infants or children during treatment with DAURISMO and for at least 30 days
after the last dose. Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about the medicines you take, including prescription medicines, over-the-counter
medicines, vitamins, and herbal supplements.
1
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

How should I take DAURISMO?
Take DAURISMO with the medicine cytarabine exactly as your healthcare provider tells you.
Take DAURISMO 1 time each day, at about the same time each day.
Take DAURISMO with or without food.
Do not chew, split or crush DAURISMO tablets.
If you miss a dose, take it as soon as you remember. If it is less than 12 hours before your next dose, just skip the
missed dose and take your next dose at your regular time. Do not take 2 doses of DAURISMO within 12 hours.
If you vomit after taking a dose of DAURISMO, do not take an extra dose, just take your next dose at your regular
time.
Your healthcare provider will perform certain tests to check you for side effects before and during treatment with
DAURISMO.
Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with DAURISMO
if you have certain side effects. Do not change your dose or stop taking DAURISMO unless your healthcare
provider tells you.
What should I avoid while taking DAURISMO?
Do not donate blood or blood products during treatment with DAURISMO and for at least 30 days after the last
dose.
Do not donate semen during treatment with DAURISMO and for at least 30 days after the last dose.
What are the possible side effects of DAURISMO?
DAURISMO can cause serious side effects, including:
See “What is the most important information I should know about DAURISMO?”
Changes in the electrical activity of your heart called QT prolongation. QT prolongation can cause irregular
heartbeats that can be life-threatening. Tell your healthcare provider right away if you feel faint, lightheaded, dizzy,
or feel your heart beating irregularly or fast during treatment with DAURISMO.
The most common side effects of DAURISMO with cytarabine include:
low red blood cell count (anemia)
low platelet count
tiredness
shortness of breath
bleeding
decreased appetite
fever with low white blood cell count
muscle pain
nausea
swellin
g
of arms or le
g
s
changes in taste
pain or sores in your mouth or throat
constipation
rash
DAURISMO may affect fertility in males. Talk to your healthcare provider if this is a concern for you.
These are not all of the possible side effects of DAURISMO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store DAURISMO?
Store DAURISMO at room temperature between 68°F to 77°F (20°C to 25°C).
Keep DAURISMO and all medicines out of the reach of children.
General information about the safe and effective use of DAURISMO.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use
DAURISMO for a condition for which it was not prescribed. Do not give DAURISMO to other people, even if they have
the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for more
information about DAURISMO that is written for health professionals.
What are the ingredients in DAURISMO?
Active ingredient: glasdegib
Inactive ingredients: microcrystalline cellulose, dibasic calcium phosphate anhydrous, sodium starch glycolate, and
magnesium stearate.
Film-coating:
25 mg tablets: Opadry II Yellow (33G120011) containing hypromellose, titanium dioxide, lactose monohydrate,
macrogol, triacetin, and iron oxide yellow.
100 mg tablets: Opadry II Beige (33G170003) containing hypromellose, titanium dioxide, lactose monohydrate,
macro
g
ol, triacetin, iron oxide
y
ellow, and iron oxide red.
2
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

LAB-1285-0.6
For more information, go to www.DAURISMO.com or call 1-800-438-1985.
This Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: November 2018
3
Reference ID: 4353045
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
