
U.S. FOOD AND DRUG ADMINISTRATION
PROPOSED ORDER (OTC000008):
AMENDING OVER-THE-COUNTER (OTC) MONOGRAPH M020:
SUNSCREEN DRUG PRODUCTS FOR OTC HUMAN USE
(Issued September 24, 2021)
I. SUMMARY
As directed by section 3854(c)(1) of the Coronavirus Aid, Relief, and Economic Security
Act (CARES Act), Public Law 116-136, 134 Stat. 281 (March 27, 2020), the U.S. Food and
Drug Administration (FDA or Agency) is issuing this proposed order to amend and revise the
deemed final administrative order concerning nonprescription sunscreen drug products
established by the enactment of the CARES Act (Deemed Final Order).
1
This Deemed Final
Order is available in the over-the-counter (OTC) Monographs@FDA portal at
https://www.accessdata.fda.gov/scripts/cder/omuf/index.cfm, where it is identified as OTC
monograph M020: Sunscreen Drug Products for Over-the-Counter Human Use.
II. LEGAL AUTHORITY
FDA is issuing this proposed order pursuant to section 505G(b) of the Federal Food,
Drug, and Cosmetic Act (FD&C Act) (21 U.S.C. 355h(b)), and section 3854(c)(1) of the CARES
Act.
III. SCOPE
This proposed order will apply to OTC sunscreen drug products governed by section
505G of the FD&C Act.
IV. BACKGROUND
OTC sunscreen drugs are topically applied products indicated to help prevent sunburn;
some are also indicated to decrease the risk of skin cancer and early skin aging caused by
exposure to the sun’s ultraviolet (UV) radiation (when used as directed with other sun protection
measures) (see § M020.50 of the Deemed Final Order). The active ingredients in sunscreen
products achieve these protective effects by absorbing, reflecting, and/or scattering radiation in
the UV range.
1
To address nonprescription sunscreen drug products that are also subject to provisions in other monographs, this
proposed order also proposes to amend and revise OTC Monograph M016: Skin Protectant Drug Products for Over-
the-Counter Human Use, and to consolidate existing and new provisions that identify sunscreens that are not
GRASE in Non-Monograph Conditions NM020: Sunscreen Drug Products for Over-the-Counter Human Use.

Proposed Order OTC000008
Page 2
In February 2019, FDA issued a proposed rule entitled “Sunscreen Drug Products for
Over-the-Counter Human Use” (2019 Proposed Rule).
2
The 2019 Proposed Rule proposed to
amend the sunscreen monograph regulation then codified in 21 CFR part 352 (which was issued
in 1999 but stayed indefinitely prior to taking effect) and to put into effect a final monograph for
sunscreens.
3
(For a detailed history of the sunscreen monograph regulation and related
rulemakings, please see pages 82 FR at 6209-6210 of the 2019 Proposed Rule). The 2019
Proposed Rule included proposals related to sunscreen active ingredients,
4
maximum sun
protection factor (SPF) levels, broad spectrum requirements, dosage forms, labeling, final
formulation testing and recordkeeping, sunscreen-insect repellent combinations, and more.
In addition, because the 2019 Proposed Rule identified a need for safety data to support
the generally recognized as safe and effective (GRASE) status of sunscreens containing certain
sunscreen active ingredients—and because FDA expected that the development of these data
could take substantially longer than the comment period on the proposed rule—the Agency
offered to consider requests to defer further rulemaking on these ingredients while the data were
being developed (see 2019 Proposed Rule at 82 FR at 6249). At the end of the comment period
on the 2019 Proposed Rule, FDA received a significant number of comments, as well as a
request to defer further rulemaking on the active ingredients avobenzone, homosalate, octinoxate,
octisalate, octocrylene, oxybenzone, ensulizole, and meradimate while data were being
developed to support their GRASE status.
2
84 FR 6204 (Feb. 26, 2019). The 2019 Proposed Rule followed from FDA’s announcement in 2011 that “we are
considering certain active ingredient safety issues further . . . . In a forthcoming rulemaking, we intend to request
additional data regarding the safety of the individual sunscreen active ingredients” (“Revised Effectiveness
Determination; Sunscreen Drug Products for Over-the-Counter Human Use” (2011 Max SPF PR), 76 FR 35672 at
35673, June 17, 2011).
3
These proposals included proposed changes to several related regulations, including labeling provisions then
codified in 21 CFR 201.327, and to new drug regulations.
4
Specifically, the 2019 Proposed Rule addressed the available data regarding safety of sunscreens containing one or
more of the following 16 active ingredients (which are the same active ingredients now addressed by the Deemed
Final Order): aminobenzoic acid (PABA), avobenzone, cinoxate, dioxybenzone, ensulizole, homosalate,
meradimate, octinoxate, octisalate, octocrylene, oxybenzone, padimate O, sulisobenzone, titanium dioxide,
trolamine salicylate, and zinc oxide. Neither the 2019 Proposed Rule nor this proposed order revisits the
contribution that these 16 active ingredients make to the effectiveness of sunscreens. Likewise, neither document
addresses sunscreen products containing the eight sunscreen active ingredients that were originally submitted under
the procedures established in 21 CFR 330.14 (the “time and extent application” (TEA) regulation)) (the TEA
ingredients) and that were the subject of proposed orders issued under section 586C of the FD&C Act (21 U.S.C.
360fff–3), established by the Sunscreen Innovation Act (SIA). (FDA’s proposed sunscreen orders on each of these
ingredients can be found at
https://www.fda.gov/drugs/guidance-compliance-regulatory-information/regulatory-
policy-information-sunscreen-innovation-act). No sponsor timely exercised the election made available by section
3854(a)(1) of the CARES Act to transition the review of such ingredient or combination of ingredients to the
process set out in section 505G of the FD&C Act. In the absence of a final order issued under the SIA finding a
TEA ingredient to be GRASE, a new drug application (NDA) is required to market sunscreens that include any of
these eight active ingredients.

Proposed Order OTC000008
Page 3
The process for amending the OTC sunscreen monograph was changed by the enactment
on March 27, 2020, of section 505G of the FD&C Act, as added by the CARES Act. Among
other things, the CARES Act replaced the rulemaking process under which the sunscreen
proposed rule had been issued with an administrative order process. In addition, section 505G
established that, as of the date of enactment of the CARES Act, a sunscreen drug that satisfies
certain requirements is deemed to be GRASE and not a new drug.
5
The CARES Act created a
“final administrative order”
for sunscreens (the Deemed Final Order) consisting of “the
requirements specified in [21 CFR 352], as published on May 21, 1999
6
. . . except that the
applicable requirements governing effectiveness and labeling [are] those specified in [21 CFR
201.327][],” which the statute established as “the applicable requirements in terms of conformity
with a final monograph” for these sunscreen drugs.
7
The CARES Act directs FDA to amend and
revise this Deemed Final Order for sunscreens, and requires that the proposed version of this
revised sunscreen order be issued not later than 18 months after the enactment of the CARES Act
(i.e., by September 27, 2021).
8
This order is being issued consistent with that requirement.
FDA proposes that the conditions laid out in the Deemed Final Order do not ensure that
sunscreen drug products are GRASE under section 201(p)(1) of the FD&C Act (21 U.S.C.
321(p)(1)) for the reasons explained in this document. This proposed order, if finalized, would
replace the Deemed Final Order in its entirety with new conditions under which nonprescription
sunscreen drug products would be determined to be GRASE under section 201(p)(1) of the
FD&C Act. It also sets forth certain characteristics that would establish that a sunscreen drug
product is not GRASE under section 201(p)(1).
5
Section 505G(a)(1)(A)(i) and 505G(a)(2); but see section 505G(m)(2) and section VI.E.i.2 of this document. We
note that before the passage of the CARES Act, sunscreens marketed without an approved application had been
marketed pursuant to an enforcement policy guidance issued in draft form in 2011 and finalized in 2018
(Enforcement Policy—OTC Sunscreen Drug Products Marketed Without an Approved Application (May 2018)).
Because the CARES Act established the requirements that sunscreens manufactured without an approved
application must follow until these requirements are revised, FDA withdrew this enforcement policy guidance in
conjunction with the posting of the Deemed Final Order. The withdrawn guidance can be viewed in FDA’s archives
at https://www.fda.gov/about-fda/about-website/fdagov-archive.
6
(1999 Final Monograph). The CARES Act specifies that these requirements begin at page 27687 of volume 64 of
the Federal Register.
7
Section 505G(a)(2) of the FD&C Act. Complementary to these requirements for conformity to the specified final
monograph, section 505G deemed the requirements of certain pre-CARES Act monograph rulemaking documents
for drugs described by the sunscreen-specific provisions of 505G(a)((2), as well as “[r]egulations in effect on the
day before the date of the enactment of [Section 505G], establishing requirements for specific nonprescription drugs
marketed pursuant to [Section 505G]” to be final administrative orders under section 505G(b)(see sections
505G(b)(8) and 505G(k)(2) of the FD&C Act). The resulting document (the Deemed Final Order) is available in the
OTC Monographs@FDA portal at https://www.accessdata.fda.gov/scripts/cder/omuf/index.cfm
.
8
See section 3854(c)(1)(B) of the CARES Act. See also section 505G(b)(8) of the FD&C Act (stating that final
monograph orders, specifically including the order consisting of the monograph establishing the conditions of use
for sunscreen under section 505G(a)(2), can be “amended, revoked, or otherwise modified in accordance with the
procedures of [section 505G(b)]).”

Proposed Order OTC000008
Page 4
In this proposed order, FDA is publishing proposed requirements that are substantively
the same as those that the Agency described in the 2019 Proposed Rule, with minor changes,
including changes to reflect the enactment of section 505G of the FD&C Act.
9
Similarly, our
scientific discussions regarding sunscreens are generally the same as those in the 2019 Proposed
Rule.
10
FDA is using this proposed order as a vehicle to efficiently transition its ongoing
consideration of the appropriate requirements for OTC sunscreens marketed without approved
applications from the previous rulemaking process to the order process created by new section
505G of the FD&C Act.
The 2019 Proposed Rule presented a thorough Agency analysis of publicly available
data regarding sunscreens at the time of its issuance. The legal and scientific standards for
general recognition of safety and effectiveness underpinning this analysis were not changed by
the CARES Act.
11
We are aware that there have been scientific developments in the time since
the proposed rule was issued, including, among other things, the publication of two new studies
on the absorption of sunscreen active ingredients,
12
both of which reinforced the need for the
sunscreen ingredient data requested in our proposed rule (and below). The comment period on
this proposed order affords an opportunity for the public to submit information that has become
available since the closure of the comment period on the 2019 Proposed Rule.
13
9
Included in these changes are our previous discussions relating to the eligibility of dosage forms, which have been
modified to reflect the passage of the CARES Act (which includes provisions relevant to this topic). Please see
Section VI.E.i.2 for further detail. Additionally, to reflect the transition to orders effectuated by section 505G, in
this proposed order, GRASE conditions for sunscreens (and for products that combine sunscreen and skin
protectants) are set forth within the relevant monographs, §§ M020 and M016, and a non-exhaustive list of
properties that render a sunscreen not GRASE are set forth in Non-Monograph Conditions § NM020. These
proposed §§ M020 and M016 provisions are substantively the same as requirements that the 2019 Proposed Rule
proposed to codify in the monograph regulations in 21 CFR parts 352 and 347, and in the labeling regulation, 21
CFR 201.327 (requirements of which the monographs proposed to incorporate by reference). We note that we have
updated our proposal for SPF testing in § M020.80 to incorporate by reference the most recent edition of an
International Organization for Standardization (ISO) standard, but that this standard is substantively unchanged from
the version previously incorporated in 21 CFR 201.327 (now part of the Deemed Final Order), which was included
in the 2019 Proposed Rule. The proposed § NM020 provisions are substantively the same as those previously
proposed (in the 2019 Proposed Rule) to be codified in 21 CFR 310.549.
10
Among other things, we have made some minor changes and corrections to our scientific discussions for
clarification and internal consistency. In addition, as indicated in the 2019 Proposed Rule, FDA defers to EPA’s
expertise and authority regarding insect repellent ingredients and has not independently evaluated those ingredients.
Accordingly, this proposed order does not include background discussion of specific insect repellant ingredients.
11
See Section 505G(k)(1) of the FD&C Act and 21 CFR 330.10(a)(4).
12
See “FDA in Brief: FDA Announces Results From Second Sunscreen Absorption Study,” available at
https://www.fda.gov/news-events/fda-brief/fda-brief-fda-announces-results-second-sunscreen-absorption-study
,
describing Matta, et al. (2020), as well as a prior pilot study (Matta et al. 2019).
13
This includes information that has become available regarding the eight sunscreen active ingredients (see footnote
4) that were the subject of timely requests for deferral in order to conduct studies to generate data first identified as
lacking in the 2019 Proposed Rule. If at any time the available evidence becomes sufficient to resolve the
uncertainty as to the GRASE status of a sunscreen containing any of these ingredients, FDA intends to proceed to a
revised final order reflecting our conclusion as to that ingredient’s status However, if at the close of the comment
period on this proposed order, the available data do not resolve the outstanding questions about each of these

Proposed Order OTC000008
Page 5
As noted above, the Agency also received a significant number of comments to the public docket
during the previous public comment period on the proposals described in the 2019 Proposed
Rule, which we continue to review. We will consider all comments that were submitted to the
public docket for the 2019 Proposed Rule within its comment period to be constructively
submitted as comments on this proposed order. To enable the Agency to review and address
these comments (and future comments that may be submitted on this proposed order) as
expeditiously as possible, we request that commenters do not re-submit comments previously
submitted on the proposed rule. FDA believes that this approach will allow us to efficiently
consider public input as the Agency assesses the appropriate regulatory requirements for
nonprescription sunscreens marketed without approved new drug applications (NDAs).
As detailed below, we emphasize that this proposed order does not represent a conclusion
by FDA that the sunscreen active ingredients included in the 1999 Final Monograph (66 FR
27666, May 21, 1999), but proposed here as needing additional data, are unsafe for use in
sunscreens. Rather, we are requesting additional information on these ingredients so that we can
evaluate their GRASE status in light of changed conditions, including substantially increased
sunscreen usage and exposure and evolving information about the potential risks associated with
these products since they were originally evaluated. As the 2019 Proposed Rule did, today’s
order also advances proposals addressing the other conditions of use for sunscreen drug products
marketed without an approved application, including broad spectrum protection, maximum SPF
requirements, dosage forms, labeling, and more.
V. SUMMARY OF MAJOR PROVISIONS OF THE PROPOSED ORDER
A. Proposed GRASE Status of Active Ingredients Listed in the 1999
Final Monograph
i. Framework for Evaluation of Safety Data
As described in further detail below, changed conditions in the time since issuance of the
1999 Final Monograph have meant that additional safety data are now needed to establish that
certain of the active ingredients listed in the 1999 Final Monograph are GRASE for use in
ingredients, but the Agency has received satisfactory indication of timely and diligent progress on the necessary
studies for a specific ingredient, FDA would be prepared to initially defer issuance of a revised final order on the
GRASE status of sunscreens containing that particular active ingredient. Such a deferral would be for a period of not
more than one year, with a possibility of extension depending on further satisfactory progress with the studies.
However, if, in FDA's judgment, studies for any active ingredient do not appear to be proceeding in a timely manner
or otherwise do not appear to be productive, the Agency expects that it will proceed to a revised final order on
sunscreens containing this ingredient after this initial deferral.

Proposed Order OTC000008
Page 6
sunscreen products in accordance with the standards established in 21 CFR 330.10(a)(4).
14
FDA’s approach to the clinical safety evaluation of OTC sunscreen active ingredients is based on
our current scientific understanding regarding the safety evaluation of topical drug products for
chronic use, and is therefore generally consistent with the safety data needed to meet the
requirements for approval of a new drug application (NDA) for a chronic-use topical drug
product (e.g., topical safety studies (irritation, sensitization, and photosafety); bioavailability
(absorption); and evaluation of adverse events observed in clinical studies). Postmarketing
safety information is also relevant to our safety evaluation.
Our current approach to the nonclinical safety evaluation of these active ingredients takes
into account their lengthy marketing history in the United States. Unlike the nonclinical data
required to meet the standard for approval of chronic-use topical NDA products (which include
comprehensive nonclinical pharmacology and toxicology safety testing), the approach to
nonclinical safety testing reflected in this proposed order is largely focused on potential long-
term adverse effects or effects not otherwise readily detected from human use (i.e.,
carcinogenicity and reproductive toxicity).
ii. Existing Safety Data for Ingredients Listed in the 1999 Final
Monograph
In section VI.C, we discuss our review of the scientific literature, submissions to the
sunscreen monograph docket, and adverse event reports submitted to FDA’s Adverse Event
Reporting System (FAERS) for the ingredients listed in the 1999 Final Monograph and identify
any existing gaps. Our review of this evidence has produced sufficient safety data on both zinc
oxide and titanium dioxide to support a proposal that sunscreen products containing these
ingredients (at concentrations of up to 25 percent) would be GRASE. Our evaluation of the
available safety data for aminobenzoic acid (PABA) and trolamine salicylate, however, has
caused us to conclude that the risks associated with use of these active ingredients in sunscreen
products outweigh their benefits. In the case of trolamine salicylate, these risks include the
potential for serious detrimental health effects (including bleeding) caused by the anti-
coagulation effects of salicylic acid and increased risk of salicylate toxicity when this ingredient
is used in sunscreens. For PABA, the risks include significant rates of allergic and photoallergic
skin reactions, as well as cross-sensitization with structurally similar compounds. Accordingly,
we are proposing that sunscreens containing these two ingredients are not GRASE due to data
demonstrating safety issues.
14
We note that certain parts of 21 CFR 330.10 establishing procedures governing the OTC drug review have been
affected by enactment of section 505G of the FD&C Act, and that appropriate regulatory changes (to withdraw
certain parts of the regulation and make corresponding technical changes) are forthcoming (see section 505G(k)(3)
of the FD&C Act). However, the standards for safety and effectiveness established in 21 CFR 330.10 remain
unchanged.

Proposed Order OTC000008
Page 7
Because the public record does not currently contain sufficient data to support positive
GRASE determinations for cinoxate, dioxybenzone, ensulizole, homosalate, meradimate,
octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, or avobenzone, we
are proposing that these ingredients are not GRASE due to insufficient data.
15
For example, the
available literature includes studies indicating that oxybenzone is absorbed through the skin to a
greater extent than previously understood and can lead to significant systemic exposure, as well
as data showing the presence of oxybenzone in human breast milk, amniotic fluid, urine, and
blood plasma. The significant systemic availability of oxybenzone, coupled with a lack of data
evaluating the full extent of its absorption potential, is a concern, among other reasons, because
of questions raised in the published literature regarding the potential for endocrine activity in
connection with systemic oxybenzone exposure. Nearly all of these sunscreen active ingredients
also have insufficient or no data characterizing their absorption.
B. Proposed Requirements Related to Dosage Forms
We are proposing the following dosage forms as GRASE for use in sunscreens: oils,
lotions, creams, gels, butters, pastes, ointments, and sticks. We are also proposing GRASE status
for spray sunscreens, subject to testing necessary to minimize potential risks from unintended
inhalation (particle size restrictions) and flammability (flammability and drying time testing),
together with related labeling requirements. We are proposing that there is insufficient data to
classify sunscreen in the powder dosage form as GRASE and expect that sunscreen powders
would also be subject to particle size restrictions if found to be GRASE in a final order. Finally,
as described in further detail in Section VI.E.i, we note that, by operation of section 505G(m)(2)
of the FD&C Act, sunscreens in all dosage forms other than the 10 dosage forms identified above
currently require an application approved under section 505 in order to be marketed (sunscreens
in dosage forms that require an NDA in order to be marketed include, for example, wipes,
towelettes, body washes, and shampoos). This order does not propose to change this
requirement.
C. Proposed Maximum Sun Protection Factor and Broad Spectrum
Requirements
In the 1999 Final Monograph, FDA established SPF 30+ as the maximum labeled SPF
value for sunscreen monograph products, and subsequently proposed (in 2011) to raise this value
to SPF 50+ (see “Revised Effectiveness Determination; Sunscreen Drug Products for Over-the-
Counter Human Use” (76 FR 35672, June 17, 2011) (2011 Max SPF PR)). The Deemed Final
Order for sunscreens established by the CARES Act does not include a limit on maximum SPF
values. Because of evidence showing meaningful clinical benefit associated with broad spectrum
sunscreen products with an SPF of 60, we are now proposing to establish a maximum labeled
15
We note that this designation generally corresponds to the Category III designation in the 2019 Proposed Rule.

Proposed Order OTC000008
Page 8
SPF value of SPF 60+. Given the lack of data showing that sunscreens with SPF values above
60 provide additional meaningful clinical benefit, we are proposing not to allow labeled SPF
values higher than 60+.
While our proposed cap for SPF labeling is SPF 60+, we are proposing to permit the
marketing of sunscreen products formulated with SPF values up to 80. This formulation margin
is intended to (1) provide formulation flexibility that we hope will help facilitate the development
of products with greater Ultraviolet A (UVA) protection and (2) more fully account for the range
of variability in SPF test results (discussed further in sections VI.E.ii.4.II-III) for sunscreen
products labeled SPF 60+. We are proposing not to allow the marketing (without an approved
NDA) of sunscreen products with SPF values above SPF 80.
In addition, since publication of the 2011 “Labeling and Effectiveness Testing; Sunscreen
Drug Products for Over-the-Counter Human Use” (L&E Final Rule) (76 FR 35620, June 17,
2011) and 2011 Max SPF PR, the body of scientific evidence linking UVA exposure to skin
cancers and other harms has grown significantly. This evidence raises concerns about the
potential for inadequate UVA protection in marketed sunscreen products—particularly in high
SPF sunscreen products that either do not pass the current broad spectrum test or (though they
pass our current broad spectrum test) have inadequate uniformity in their UVA protection.
Consumers using these products may, while successfully preventing sunburn, accumulate
excessively large doses of UVA radiation—thereby exposing themselves to additional risks
related to skin cancer and early skin aging.
To address these concerns, we are making a number of proposals designed to couple a
greater magnitude of UVA protection to increases in SPF values. We are proposing to require
that all sunscreen products with SPF values of 15 and above satisfy broad spectrum
requirements. Among other things, this proposal eliminates the potential confusion permitted by
the current labeling regime, in which a higher numbered product (for example, one labeled SPF
30) may provide inferior protection against UVA radiation than a lower numbered product (for
example, one labeled broad spectrum SPF 15). We are also proposing to add to the current broad
spectrum test a requirement that broad spectrum products meet a UVA I/UV ratio of 0.7 or
higher. Given how much of the UVA portion of the ultraviolet (UV) spectrum is composed of
UVA I radiation, and given what we now know about the skin cancer risks associated with UVA
exposure, ensuring that sunscreen products provide adequate protection in the UVA I portion of
the spectrum is critical.
16
Because sunscreens with SPF 2 to 14 have not been demonstrated to
16
We note that because our proposal to raise the maximum labeled SPF value to 60+ is based on studies that all used
broad spectrum sunscreens, the additional clinical benefit we are proposing to recognize in sunscreen products with
SPF values greater than 50 cannot be decoupled from the broad spectrum protection provided by those products. As
a result, our proposal to raise the maximum labeled SPF value to SPF 60+ is both consistent with and dependent
upon our proposal to require that all sunscreen monograph products with SPF values of 15 and above satisfy our
broad spectrum requirements.
Proposed Order OTC000008
Page 9
help reduce the risk of skin cancer and early skin aging caused by the sun, whether or not they
provide protection against UVA radiation as well as ultraviolet B (UVB) radiation, we are not
proposing to require that they pass the revised broad spectrum test. However, we seek comment
on whether these low SPF products should remain in the market.
Finally, we are proposing to require that sunscreen products with SPF values of 15 or
above be labeled with an SPF number corresponding to the lowest number in a range of tested
SPF results. For example, sunscreens testing at SPF 15-19 would be labeled “SPF 15”; those
testing at 40-49 would be labeled “SPF 40.” We are making this proposal because new evidence
has caused us to reexamine the variability inherent in the SPF test (which relies on visual
assessments of erythema in human subjects). The data we reviewed suggests that the clinical
evaluation undertaken during SPF testing creates variability that justifies the use of SPF ranges.
As explained further in sections VI.E.ii.4.II-III, because this variability is exacerbated at high
SPFs, we are proposing that sunscreens testing at SPF 30 or more be labeled in increments of 10
(i.e., SPF 30, SPF 40, SPF 50, with a proposed maximum of SPF 60+), that sunscreens testing at
SPF 15 to 29 be labeled in increments of 5 (i.e., SPF 15, SPF 20, SPF 25), and that the
requirement that labeled SPF values correspond to ranges (rather than precise numerical values)
is not necessary below SPF 15.
D. Proposed PDP Labeling Requirements
We are also proposing to partially revise the current requirements for information that
must appear on the principal display panel (PDP) of sunscreen products. The PDP is the part of a
product label that is most likely to be viewed or examined when the product is displayed for
retail sale. A major feature of the PDP is the statement of identity (SOI). We are proposing that
the SOI consist of an alphabetical listing of the sunscreen active ingredients in the product,
followed by “Sunscreen” and the product’s dosage form (such as lotion or spray). This
information would supplement other important elements of the PDP (e.g., SPF, broad spectrum,
and water resistance information) to provide a succinct summary of the product’s key
characteristics on the front of the package or container, permitting consumers to more readily
compare products and either select or avoid a given product accordingly. For sunscreen products
that have not been shown to help prevent skin cancer or early skin aging caused by the sun, the
SPF statement would be followed by an asterisk (*) directing consumers to see the “Skin
Cancer/Skin Aging alert” elsewhere on the label. Finally, to prevent required information from
being obscured or overwhelmed by other labeling features, we are revising the format
requirements for the SPF, broad spectrum, and water resistance statements on the PDP.
E. Proposed Requirements Related to Final Formulation Testing
Processes and Recordkeeping

Proposed Order OTC000008
Page 10
To ensure that nonprescription sunscreen products are marketed under GRASE
conditions, we are proposing to require records of required final formulation testing of sunscreen
products to be maintained for 1 year after the product expiration date, or, if the product is exempt
from expiration dating (as most sunscreens are), for 3 years after distribution of the last lot
labeled in reliance on that testing. In addition, we are proposing to require responsible persons
(defined in section VI.E.iv.2.II) to keep records of sunscreen formulation testing, and we are
clarifying that required records would be subject to FDA inspection. We are also proposing a
number of revisions to the labeling and testing requirements for nonprescription sunscreens that
are designed to clarify FDA expectations about clinical final formulation testing processes and to
ensure that the testing of marketed sunscreen products is conducted in a manner that both
protects human subjects and produces reliable results.
F. Proposed Status of Sunscreen-Insect Repellent Combination
Products
17
The proposed order also addresses sunscreen-insect repellent products, which are jointly
regulated by FDA as sunscreen drugs and by the U.S. Environmental Protection Agency (EPA)
as pesticides under the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA). In 2007,
FDA and EPA both issued Advance Notice of Proposed Rulemakings (ANPRs) requesting
comment on the appropriate regulatory status of these products. We are proposing to classify
these products as not GRASE because incompatibilities between FDA and EPA labeling
requirements prevent these products from being labeled in a manner that sufficiently ensures safe
and effective use of the sunscreen component. In addition, there are data suggesting that
combining some sunscreen active ingredients with the insecticide N,N-Diethyl-meta-toluamide
(DEET) may increase absorption of either or both components.
18
G. Other Proposals for Harmonization and/or Consolidation
To harmonize the requirements for products that contain both sunscreen and skin
protectant active ingredients, we are proposing changes in both § M016, Skin Protectant Drug
Products for Over-the-Counter Human Use,
19
(skin protectant monograph) and the sunscreen
monograph (§ M020). In addition, we propose to consolidate under new Non-Monograph
17
“Use of the descriptor “combination product(s)” in this document is not intended to convey that a product
containing one or more sunscreen active ingredients together with one or more insect repellents is a “combination
product” as that term is defined in 21 CFR 3.2(e).
18
See Ross et al 2004; Gu et al. 2005; Wang and Gu 2007; Kasichayanula et al. 2005; Kasichyanula et al. 2007;
Yiin et al. 2015.
19
Over-the-Counter M016: Skin Protectant Drug Products for Over-the-Counter Human Use is a final administrative
order that, as deemed by section 505G(b)(8) of the FD&C Act, incorporated the requirements of the final
monograph for OTC skin protectant drug products issued under 21 CFR part 330, as codified in 21 CFR part 347 as
of March 27, 2020 and the proposed rules issued in the Federal Register on January 31, 1990 (55 FR 3362) and June
20, 1990 (55 FR 25204), with technical amendments. It is available at OTC Monographs@FDA portal at
https://www.accessdata.fda.gov/scripts/cder/omuf/index.cfm
.

Proposed Order OTC000008
Page 11
Conditions NM020: Sunscreen Drug Products for Over-the-Counter Use both the existing
sunscreen provisions from Non-Monograph Conditions § NM900
20
and the new characteristics
proposed in this order as properties that render an OTC drug product offered for use as sunscreen
not GRASE under section 505G(b)(1)(C) of the FD&C Act. Therefore, a sunscreen product with
any characteristic in new § NM020 would be a new drug under section 201(p) of the FD&C Act
(for which an approved NDA is required prior to marketing).
VI. REASONS FOR ISSUANCE
A. Need for Additional Safety Information
i. Increased Consumer Exposure to Sunscreen Active Ingredients
Consumer exposure to sunscreen active ingredients has increased dramatically since FDA
began its initial safety evaluations of the sunscreen active ingredients at issue in this proposed
order in the 1970s. Many factors have influenced this increase, including the following:
• Significant increases in the number and types of consumers using sunscreen
products (Matlack 2009; National Cancer Institute 2018)
• Sunscreen products containing a greater number of active ingredients at
greater concentrations (Urbach 2001)
• Increased awareness of the risks of sun exposure and encouragement of
routine sunscreen use by medical and public health authorities (see, e.g., U.S.
Department of Health and Human Services, no date (n.d.))
• Evolving directions for use on sunscreen products instructing consumers to
use greater amounts of sunscreen per application and to reapply sunscreen
products more frequently (76 FR 35672 at 35678) (see Deemed Final Order at
§ M020.50)
• Expanding availability and use of many different types of sunscreen products,
including daily-use products such as facial makeup, moisturizing creams, and
lipstick
Relatively few sunscreen products were in use when the U.S. Army initially funded
research into the development of effective sunscreen products for use by military personnel on
aircraft carriers (and others routinely exposed to long periods of intense sunlight) during World
War II (Matlack 2009). The reach of sunscreen products began to broaden when they were later
20
Non-Monograph Conditions NM900: Drug Products Containing Certain Active Ingredients Offered Over-the-
Counter for Certain Uses, encompassed the provisions of 21 CFR 310.545 as in effect on March 26, 2020, which
were deemed to be a final administrative order by section 505G(k)(2)(A) of the FD&C Act upon its enactment. It is
available in the OTC Monographs@FDA portal at https://www.accessdata.fda.gov/scripts/cder/omuf/index.cfm
.

Proposed Order OTC000008
Page 12
marketed for use specifically by consumers who sunburned readily (i.e., fair-skinned individuals)
in situations of intentional sun exposure, such as sunbathing on a beach (Svarc 2015). Sunscreen
products are now routinely used by a much broader range of consumers for protection against
many types of sun-induced skin damage, not just sunburn. Accumulating data demonstrate that
increased sun exposure increases the risk of developing skin cancers and premature skin aging
(Matlack 2009). To help reduce the risk of these types of sun-induced skin damage, public
health organizations (including FDA) have for years urged consumers to use sunscreen products
along with other sun-protective behaviors like limiting time in the sun and wearing protective
clothing (FDA 2019b; Centers for Disease Control and Prevention, n.d.; American Cancer
Society, n.d.).
Another factor driving increased consumer exposure to sunscreen active ingredients has
been the introduction and widespread adoption of sunscreen products with higher labeled SPF
values. The maximum SPF value proposed for sunscreen labeling has progressively increased
from SPF 15 in the 1978 report
21
issued by the advisory panel convened by FDA to evaluate the
safety and efficacy of sunscreens, to SPF 30+ in the 1999 Final Monograph, to SPF 50+ in the
2011 Max SPF PR. To achieve these higher SPFs, many currently marketed products are
formulated with more active ingredients combined together in higher concentrations than were
generally combined in products when FDA’s review of OTC sunscreens began. Increased
knowledge about the role of UVA radiation in causing skin damage has also encouraged the
formulation of broad-spectrum products with combinations of active ingredients designed to
achieve protection against both UVA and UVB radiation. In addition, other widely used
products, such as facial makeup, moisturizing creams, and lipsticks have had sunscreen active
ingredients added to their formulations. These trends are reflected in the evolution of the current
labeling provisions for sunscreen products regulated under the OTC monograph system.
Changes in the instructions for using these sunscreen products have also contributed to
increased use of, and exposure to, sunscreen active ingredients. The labeling recommended in
1978 by the advisory panel simply instructed consumers to apply sunscreen products liberally
and to reapply after swimming or excess perspiration (43 FR 38206 at 38215). Current labeling,
by contrast, encourages consumers to regularly use a broad spectrum SPF 15 or higher sunscreen
product with other sun protection measures, and to apply all sunscreens generously/liberally 15
minutes before sun exposure and reapply at least every 2 hours or more frequently when
swimming or sweating (see Deemed Final Order at § M020.50(e)).
ii. Emerging Safety Concerns
In recent years, a growing body of data has suggested that the transdermal absorption of
some sunscreen active ingredients is greater than previously thought, and thus may raise
21
43 FR 38206 (August 25, 1978).
Proposed Order OTC000008
Page 13
previously unevaluated safety concerns, including the potential for reproductive, developmental,
or carcinogenic effects. As discussed in further detail in section VI.C.iii.2.I, newly available
information suggests, for example, that there is the potential for toxicity associated with the
transdermal absorption and systemic availability of oxybenzone. This new information about
absorption and potential safety risks is inadequate, by itself, to support an affirmative conclusion
that products containing the active ingredients at issue are not safe. Coupled with the lack of
clinical pharmacology and nonclinical safety data for certain sunscreen active ingredients,
however, it leads us to conclude that, for some sunscreen active ingredients, the current record
does not include adequate evidence of safety to satisfy the applicable legal standards for general
recognition of safety and effectiveness as set forth in 21 CFR 330.10.
B. Framework for Evaluation of Safety Data
In light of these safety concerns, FDA held a meeting of its Nonprescription Drugs
Advisory Committee (NDAC) on September 4 and 5, 2014, to discuss the scope of safety testing
that should be conducted to support general recognition of safety and effectiveness for active
ingredients for use in nonprescription sunscreen products. FDA proposed the following safety
testing paradigm:
Clinical data:
• Dermal irritation and sensitization testing
• Phototoxicity and photoallergenicity testing
• Human maximal use bioavailability studies
• Postmarketing adverse event reports
Nonclinical (toxicology) data:
• Dermal carcinogenicity
• Systemic carcinogenicity
• Developmental and reproductive toxicity (DART)
• Toxicokinetics (including absorption, distribution, metabolism, and excretion)
• Additional testing when data suggest a concern about other long-term effects,
such as endocrine effects
There was consensus among the committee members that FDA’s proposed framework was a
good starting point (FDA 2014b). In November 2015, FDA published a draft guidance for
industry Over-the-Counter Sunscreens: Safety and Effectiveness Data (see 80 FR 72975,
November 23, 2015),
which described and requested comment on the safety and effectiveness
data necessary to determine whether an OTC sunscreen active ingredient or combination of
active ingredients evaluated under the SIA was GRASE when used under specified conditions.

Proposed Order OTC000008
Page 14
FDA finalized this guidance in November 2016, after considering public comment on its draft
recommendations (FDA 2018b).
22
The recommendations in this guidance reflect FDA’s
scientific expertise, existing technical guidance, experience from reviewing safety and efficacy
data submitted for GRASE review of sunscreen active ingredients under the OTC Drug Review,
and input from and concurrence by outside scientific experts.
All sunscreens marketed without an NDA are subject to the same standard: general
recognition of safety and effectiveness. Accordingly, the data that we expect to be necessary to
evaluate the safety and effectiveness of the sunscreen monograph active ingredients are the same
as those we recommended as necessary to evaluate the safety and effectiveness of sunscreen
active ingredients originally submitted under the procedures established in 21 CFR 330.14
23
(the
“time and extent application” or “TEA” regulation) and that were the subject of proposed orders
issued under section 586C of the FD&C Act (21 U.S.C. 360fff-3) as established by the SIA (see
the guidance for industry Nonprescription Sunscreen Drug Products — Safety and Effectiveness
Data (FDA 2016)).
The studies described in this section are generally needed for FDA to determine that a
sunscreen active ingredient is GRASE for use in nonprescription sunscreens. Specific data gaps
for individual active ingredients depend on the quality and quantity of available safety data and
are identified in section VI.C. As described in that section, FDA proposes that sunscreens
containing active ingredients for which the existing public record contains sufficient data to
support a positive GRASE finding are GRASE under section 201(p)(1) of the FD&C Act (and
are not subject to section 503(b)(1) of the FD&C Act (21 U.S.C. 353(b)(1)) provided that they
satisfy all the other conditions specified in a final sunscreen order. See section 505G(b)(1)(A).
The Agency proposes that sunscreens containing active ingredients for which additional data are
necessary are not GRASE under section 505G(b)(1)(C)(ii) of the FD&C Act because “the
evidence is inadequate to show that [these sunscreens are] generally recognized as safe and
effective under section 201(p)(1) [of the FD&C Act].” Finally, in evaluating the existing safety
data for the active ingredients listed in the 1999 Final Monograph, FDA determined that the risks
associated with two of these ingredients (PABA and Trolamine Salicylate) outweigh their
benefits. As discussed in further detail in section VI.C.ii, FDA is therefore proposing that
sunscreens containing these two ingredients are not GRASE under section 505G(b)(1)(C)(i) of
the FD&C Act because the evidence shows that these sunscreens are not generally recognized as
safe and effective under section 201(p)(1) of the FD&C Act.
22
FDA’s recommendations regarding the safety and effectiveness data necessary to determine whether an OTC
sunscreen active ingredient (or combination of ingredients) evaluated under the SIA was GRASE when used under
specified conditions generally remained unchanged in the final guidance.
23
We note that 21 CFR 330.14 has been affected by enactment of section 505G of the FD&C Act, and that
appropriate regulatory changes are forthcoming. See section 505G(k)(3) of the FD&C Act.

Proposed Order OTC000008
Page 15
i. General
FDA’s OTC drug regulations identify the general types of safety information that should
be submitted as evidence that an OTC drug is GRASE for use as labeled and the standard by
which safety is to be judged (21 CFR 330.10(a)). When applying these regulations to each drug,
FDA uses its scientific expertise to determine what constitutes “adequate tests by methods
reasonably applicable to show the drug is safe under the prescribed, recommended, or suggested
conditions of use” (21 CFR 330.10(a)).
FDA recognizes the contribution that broad spectrum sunscreens with an SPF value of 15
or higher can make to decreasing the risk of skin cancer and early skin aging caused by the sun if
used as directed with other sun protection measures. To protect the public health, however, it is
also important for FDA to balance the potential benefits of these sunscreen products to
consumers against their potential risks. Providing an adequate safety margin for OTC sunscreen
active ingredients and finished sunscreen products is a key element of FDA’s risk assessment. A
safety margin calculation takes the lowest animal NOAEL (no observed adverse effect level) and
compares it to exposure for humans.
24
Because animal studies do not always predict effects in
humans, the actual threshold for an effect in humans may be different (i.e., higher or lower) than
in the species tested. The human sensitivity to a drug is often unknown. To account for this,
FDA will determine if the calculated safety margin is adequate, considering the toxicities seen in
animals.
In determining the specific testing and other data needed to adequately demonstrate that
an OTC sunscreen active ingredient is safe, FDA considers both the circumstances under which
OTC sunscreen products are intended to be used by consumers (i.e., the conditions of use) and
current scientific knowledge and assessment technology. FDA’s approach to the clinical safety
evaluation of OTC sunscreen active ingredients is based on our current scientific understanding
regarding safety evaluation of topical drug products for chronic use, and thus is generally
consistent with the safety data requirements that would apply to an NDA for a chronic-use
topical drug product (i.e., topical safety studies (irritation, sensitization, and photosafety);
bioavailability (absorption); and evaluation of adverse events observed in clinical studies).
25
In
addition, the evaluation of adverse events reported during the commercial marketing of
sunscreen products containing the ingredient and other postmarketing safety information is also
relevant to safety.
24
This description of the calculation of a safety margin has been corrected from what is reflected in the 2019
Proposed Rule and 2016 guidance for industry Nonprescription Sunscreen Drug Products — Safety and
Effectiveness Data (FDA 2016).
25
Chronic use is defined as continuous or intermittent use for at least 6 months during the course of a lifetime.
Proposed Order OTC000008
Page 16
FDA’s approach to the nonclinical safety evaluation of these active ingredients takes into
account their lengthy marketing history in the United States. In contrast to nonclinical data
requirements for a chronic-use topical drug product NDA, which include results from
comprehensive nonclinical pharmacology and toxicology safety testing, the approach to
nonclinical safety testing in this proposed order is largely focused on potential long-term adverse
effects or effects not otherwise readily detected from human use (i.e., carcinogenicity and
reproductive toxicity). Additional testing beyond what is described below may be recommended
for active ingredients for which data suggest a concern about other long-term effects, such as
hormonal disruption.
In addition, although sunscreen products are typically formulated with two or more active
ingredients, the framework described below contemplates that testing will be performed using
formulations that include one active ingredient. Generally, unless data suggest that there may be
a safety or efficacy concern with a particular combination of active ingredients, we anticipate
that an active ingredient that is found to be GRASE for use in sunscreens could be combined
with other active ingredients that are also GRASE for use in sunscreens. If data suggest that
there may be a safety or efficacy concern with a particular combination of active ingredients (or
active and inactive ingredients), additional data may be necessary to support a positive GRASE
determination for sunscreens containing that combination.
The following sections describe the specific safety data that FDA expects the Agency
will need to determine whether an active ingredient is GRASE for use in sunscreens.
ii. Clinical Safety Testing
1. Human Dermal Safety Studies
Human dermal safety studies for topical products in which exposure to light after
application is anticipated generally consist of two sets of studies—those conducted without
specific exposure to light and those conducted to assess reactions after UV exposure (photosafety
studies) (FDA 2015b). The studies usually consist of dermal irritation patch testing, dermal
sensitization patch testing, dermal phototoxicity testing, and dermal photoallergenicity testing.
Because marketed sunscreen products typically contain a combination of active
ingredients, and product formulations frequently change, it is difficult to determine causal links
between individual active ingredients and reported irritation and hypersensitivity adverse events
associated with a particular product. Therefore, FDA generally expects to use data from human
dermal irritation studies, human dermal sensitization studies, and human dermal photosafety
studies, in conjunction with postmarketing adverse event data, to inform GRASE determinations
and labeling. Nonetheless, in some cases, it may be reasonable to omit human dermal irritation
studies, human dermal sensitization studies, and/or human dermal photosafety studies, depending
Proposed Order OTC000008
Page 17
on the rigor of available postmarketing safety information. For example, if FDA concludes that
there is a positive risk-benefit profile for a sunscreen active ingredient, but that it is known to be
a sensitizer, it may be possible to develop safety labeling to address this risk without data
generated in the human dermal safety studies described below (see, e.g., section VI.C.iii.2.I.v).
I. Human dermal irritation and sensitization
studies
Studies of dermal irritation and sensitization, using the repeat insult patch test or other relevant
tests, are elements in the safety evaluation of topical drug products that, like sunscreens, are
applied to the skin repeatedly over long periods of time. Designed to detect the potential for
local dermatologic events with fewer subjects than might be observed in larger clinical trials,
these tests often employ product application that is more frequent and/or for longer duration than
proposed clinical dosing. In dermal irritation studies, a test substance is applied to a small pad
(patch) and affixed to the test subject’s skin, usually on the back, to determine whether the
ingredient causes direct skin toxicity. Dermal sensitization studies are conducted similarly but
are designed to detect immunologically mediated reactions, which require prior exposure to the
allergen.
Nonprescription sunscreens regulated under the OTC monograph system may be used in
many product formulations, including those yet unknown. Therefore, cumulative irritation
studies that evaluate the sunscreen active ingredient at the highest concentration for which a
GRASE determination is sought should be conducted using the ingredient in an appropriate
vehicle, using the vehicle alone, and using both negative and positive controls. The evaluation
should include scoring of erythema, edema, and a papular response or skin erosion.
Dermal sensitization studies, conducted to detect immunologically mediated reactions,
should be conducted in three phases: (1) the induction phase (3 weekly applications for 3
weeks); (2) the rest phase (no product application for 10 to 14 days); and (3) the challenge phase
(patch applications to new sites for 48 hours with a confirmatory rechallenge to exclude false
positives).
Although FDA recommends separate dermal irritation and sensitization studies, it may be
appropriate to combine irritation and sensitization studies in the same study as long as a
sufficient number of subjects are included for sensitization evaluation.
II. Human photosafety studies
Topically applied dermatologic drug products should be tested for photosafety if they absorb
light in the UVA, UVB, or visible spectra. Photosafety evaluations of sunscreen active
ingredients that absorb light should consist of skin photoallergenicity and skin phototoxicity
testing. Photoallergy is an immunologically mediated reaction to a chemical, initiated by the

Proposed Order OTC000008
Page 18
formation of photoproducts (e.g., protein adducts) following a photochemical reaction. Similar
to dermal sensitivity testing described above, photoallergy tests use an
induction/rest/challenge/rechallenge multiphase design to assess erythema, edema, and
vesiculation. Phototoxicity (or photoirritation) is an acute light-induced tissue response to a
photoreactive chemical. Phototoxicity testing typically includes a test patch, a vehicle patch, and
a sham patch application for 24 hours, followed by UV light exposure of the test area. A second
set of patch application areas not irradiated with light serves as a control. FDA expects that, to
support a GRASE finding, photosafety studies of sunscreen active ingredients that absorb light
will need to be conducted using the active ingredient at the highest concentration for which a
GRASE determination is sought in an appropriate vehicle, using the vehicle alone, and with a
negative control.
2. Human Absorption Studies/Maximal Usage Trial
Because nonprescription sunscreens are topically applied, a critical safety consideration
is whether dermal application results in skin penetration and systemic exposure to their active
ingredients and, if so, to what extent. This information helps identify potential safety concerns
and helps determine whether an adequate safety margin exists within which an active ingredient
is GRASE for use in sunscreens.
The principal barrier to topical drug product penetration is the multilayered, lipid-rich
stratum corneum. The passage of any drug product through this layer is influenced by many
factors, including the drug product’s physicochemical features, molecular weight, and
vehicle/formulation properties. Vehicle/formulation properties are particularly important
because the choice of vehicle can markedly affect the permeation potential of a drug product.
Effects can range from simple hydration of the stratum corneum by occlusive
vehicles/formulations to direct permeation enhancement by solvent effects on the lipids in the
stratum corneum. Products absorbed through the skin have the potential to cause systemic
adverse effects, affecting the safety assessment. Because sunscreens are intended to work at the
skin’s surface, systemic absorption may also lower efficacy, affecting the efficacy assessment.
Such considerations ultimately weigh into the risk-benefit calculus FDA uses to determine
whether an OTC sunscreen containing a given active ingredient is GRASE.
Since the mid-1990s, topical product NDAs have included a Maximal Usage Trial
(MUsT) program as part of the clinical pharmacology/bioavailability assessment. A MUsT
program is designed to capture the effect of maximal use on absorption into the blood with
standard pharmacokinetic assessments (e.g., C
max
, T
max
26
, area under the curve, half-life,
clearance, and volume of distribution) (for further information about conduct of a MUsT
program, see Bashaw et al. 2015). For a topical product NDA, the MUsT program is usually
conducted in subjects with the disease of interest, where disrupted skin is a feature. In situations
26
C
max
is the peak plasma concentration and T
max
is the time to peak plasma concentration.

Proposed Order OTC000008
Page 19
where disrupted skin is not a feature of the condition being treated or the topical product is
intended for prevention of disease (e.g., sunscreens), the MUsT program for a topical product
NDA should be conducted in subjects with healthy, intact skin. The MUsT program for a topical
product NDA is conducted with the specific product formulation for which approval is sought
applied at the upper limit of surface area involvement that is studied in the phase 3 clinical trials
and is proposed for labeling. For example, if the proposed labeling of an acne product permits
the product to be used on up to 30 percent of body surface area, that would be the coverage
evaluated in the MUsT program.
We expect that data from a MUsT program will be needed to support an adequate
assessment of safety for most sunscreen active ingredients FDA 2014b). Because sunscreen
products regulated pursuant to the OTC monograph system may include active ingredients in a
variety of formulations, FDA recommends that a MUsT program be conducted under maximal
use conditions employing a minimum of four formulations, containing the sunscreen active
ingredient as the only active ingredient.
27
These formulations should be prepared using
vehicle/formulation systems that are appropriate for sunscreen topical products (e.g., they are
deployable and spreadable) that represent real-world marketed formulations, and that are
expected to produce the highest in vivo absorption. Justification for the formulations chosen,
including results of in vitro testing using a human cadaver skin permeation system (e.g., static
cell, also known as vertical diffusion cell) (Bassani et al. 2017; U.S. Pharmacopeia 2017), should
be included in the study protocol. The protocol should contain sufficient detail for others to
reproduce the formulations and manufacturing process.
28
FDA anticipates that the use of multiple formulations will help identify the overall
absorption potential of the sunscreen active ingredient of interest. The MUsT program should be
conducted in subjects with healthy, intact skin
29
at the highest concentration of the ingredient for
which a GRASE determination is sought. Based on recommended sunscreen use on all exposed
skin, the exposed area should include at least 75 percent of the body surface area. Data from the
formulation that produces the highest in vivo absorption would then be used to determine the
safety margin.
27
We note, however, as described in section VIII.C.1.b, that because of avobenzone’s potential for
photodegradation, we recommend that a MUsT program for avobenzone evaluate avobenzone in combination with a
photostabilizer. In some cases, sunscreen active ingredients (e.g., octocrylene) can serve as photostabilizers. In
such cases, we expect that the MUsT program could include such ingredients.
28
FDA has issued guidance with recommendations for the conduct of MUsT studies to support the safety of active
ingredients that are candidates for inclusion in a topical drug product under an OTC Drug monograph (see the
guidance for industry Maximal Usage Trials for Topically Applied Active Ingredients Being Considered for
Inclusion in an Over-The-Counter Monograph: Study Elements and Considerations (FDA 2019a)). FDA also
encourages persons who are interested in conducting a MUsT to support the safety of an active ingredient to discuss
proposed protocols with the Agency.
29
As discussed infra, the MUsT should be conducted on healthy, intact skin because sunscreens are intended for
prevention rather than treatment.

Proposed Order OTC000008
Page 20
The assay used in the MUsT program should be properly validated according to current
good laboratory practices (21 CFR part 58). Additionally, the Agency’s most current guidance
on bioanalytical method validation may be found by searching at
https://www.fda.gov/RegulatoryInformation/Guidances/default.htm. The assay’s limit of
quantitation-limit of detection should be sufficiently low to allow a signal-to-noise ratio that
ensures confidence in detection of a concentration of 0.5 nanogram (ng)/milliliter (mL) for the
compound of interest. In other words, for sunscreen active ingredients, FDA expects that the 0.5
ng/mL concentration will be sufficiently above the assay’s limit of quantitation-limit of detection
to allow a signal-to-noise ratio that ensures confidence in either the detected concentrations or
lack of concentrations.
An important consideration for designing a MUsT program is that it should include
testing for a duration that allows for the attainment of steady state levels to ensure that maximum
penetration of the ingredient has taken place and to optimize the chances of the ingredient being
detected. Thus, for sunscreen active ingredients, FDA expects that single application studies
would be inadequate. Because the subjects in a MUsT program represent an enriched dataset in
the upper range of exposures, safety-related data (such as vital signs, adverse events) from the
study’s regularly scheduled physical examinations should also be collected. We strongly
encourage consultation with FDA about MUsT protocols before beginning the trial.
Finally, as discussed further in section VI.C.4, if the sunscreen active ingredient is
determined to be GRASE for use in sunscreens, the sunscreen monograph, when finalized, must
set out the conditions under which any future sunscreen containing that active ingredient will be
GRASE and not misbranded. As such a condition, FDA is considering certain final formulation
testing to address the potential for transdermal absorption and its impact on safety. FDA
anticipates that the formulation that produces the highest in vivo absorption in the MUsT
program would be appropriate to designate as a standard control formulation for future in vitro
human cadaver skin permeation system testing (e.g., a static or vertical diffusion cell) of each
final sunscreen formulation that includes that active ingredient. If such testing were included as
a condition in a final sunscreen monograph, and if in vitro permeation of the sunscreen active
ingredient in the final product formulation were equal to or less than the value from in vitro
testing of the standard control formulation (that was shown by the MUsT program to have the
highest degree of systemic absorption), FDA anticipates that the safety margin previously
calculated would be considered adequate to support the safety of the finished formulation.
3. Pediatric Considerations
Young children have a larger ratio of skin surface to body volume than adults, which can
increase a child’s systemic exposure to topically applied drug products. In addition, growing
Proposed Order OTC000008
Page 21
children have greater potential to experience deleterious developmental effects from drug
exposure. If the calculated safety margin for an active ingredient (based on nonclinical results
and human MUsT program) is relatively small, FDA will exercise its scientific judgment to
determine whether a sunscreen active ingredient MUsT program in young children or other
studies are warranted to ensure that the safety margin for marketed products containing the
ingredient is within an acceptable range for this population.
iii. Nonclinical Safety Testing
1. Carcinogenicity Studies: Dermal and Systemic
FDA generally recommends carcinogenicity studies for any pharmaceutical with an
expected clinical use (either intermittent or continuous) of at least 6 months (FDA 1996). The
animal carcinogenicity studies help characterize the potential tumor risks associated with use of a
sunscreen active ingredient in human beings by identifying any observed tumors by type, the
level of exposure at which tumors occur, and the highest level of exposure at which no adverse
effects occur, referred to as the NOAEL. As noted earlier, FDA intends to use the NOAEL in
determining the safety margin for human exposure to sunscreens containing the active
ingredient. In addition to detecting carcinogenic potential, carcinogenicity studies in animals can
also help to identify other systemic or organ toxicities that may be associated with the sunscreen
active ingredient.
FDA expects that a dermal carcinogenicity study involving application of the test article
to the skin of mice or rats for 2 years will thus need to be conducted to support a GRASE finding
for the active ingredient unless the ingredient has been demonstrated not to reach the viable
layers of the skin where it could impact skin tumor development. FDA also considers it
important to study the effects of systemic exposure if human bioavailability data show that
dermal application of a particular formulation results in skin penetration and systemic exposure
to the active ingredient or related compounds (including metabolites). Therefore, we expect that
a second carcinogenicity study by a route that produces systemic exposure will also be needed to
support the safety of a sunscreen active ingredient, if systemic exposure is observed in the
bioavailability data, or if nonclinical and clinical studies (including an adequately conducted
toxicology program) reveal any other safety signals for the ingredient or any known structurally
similar compounds, including metabolites. This can be a 2-year study or a shorter (usually 6
months) alternative carcinogenicity model, and it should be conducted in a species different from
that used in the dermal carcinogenicity study. FDA notes that the absence of a carcinogenicity
signal from an alternative transgenic carcinogenicity study (e.g., TgRasH2 mouse) would likely
support the safety of a sunscreen active ingredient. If a carcinogenicity signal were observed in
such a study, however, the study could not be used to support the safety of a sunscreen active
ingredient because there would be no basis for calculating a safety margin with this study

Proposed Order OTC000008
Page 22
(Jacobs and Brown 2015). All carcinogenicity studies, regardless of route, should assess a full
panel of tissues.
30
FDA expects that a systemic carcinogenicity study would not be needed to support a
GRASE determination for a sunscreen active ingredient if an adequately conducted human
pharmacokinetic MUsT program resulted in a steady state blood level less than 0.5 ng/mL, and
an adequately conducted toxicology program did not reveal any other safety signals for the
ingredient or any known structurally similar compounds, including metabolites, indicating the
potential for adverse effects at lower levels. The threshold value of 0.5 ng/mL is based on the
assessment that the level would approximate the highest plasma level below which the
carcinogenic risk of any unknown compound would be less than 1 in 100,000 after a single dose.
This threshold value is consistent with the Threshold of Toxicological Concern concept.
31
For
sunscreen active ingredients, FDA expects that the 0.5 ng/mL concentration will be sufficiently
above the assay’s limit of quantitation—limit of detection to allow a signal-to-noise ratio that
ensures confidence in either the detected concentrations or lack of concentrations.
2. Developmental and Reproductive Toxicity Studies
FDA expects that DART studies will need to be conducted to evaluate the potential
effects that exposure to the sunscreen active ingredient may have on developing offspring
throughout gestation and postnatally until sexual maturation, as well as on the reproductive
competence of sexually mature male and female animals (FDA 2021). As with systemic
carcinogenicity studies, we expect that studies to assess fertility and early embryonic
development, and pre- or postnatal toxicity in rats will not be needed if an adequately conducted
human MUsT program shows a steady state blood level less than 0.5 ng/mL, and an adequately
conducted toxicology program produces no signals indicating that the ingredient (including its
clinically relevant metabolites) or any known structurally similar compound, including a
metabolite, interacts with related pathways.
32
We expect that effects on embryofetal
development will need to be assessed in rats and rabbits in all cases.
30
FDA recommends submitting the carcinogenicity study protocol(s) for review by FDA’s Center for Drug
Evaluation and Research’s (CDER’s) Executive Carcinogenicity Assessment Committee before initiating the
studies. For further guidance regarding carcinogenicity studies, see the FDA guidance for industry Carcinogenicity
Study Protocol Submissions” (FDA 2002).
31
This threshold concept was applied to impurities in the International Council for Harmonization of Technical
Requirements for Pharmaceuticals for Human Use (ICH) guidance for industry M7(R1) Assessment and Control of
DNA Reactive (Mutagenic) Impurities in Pharmaceuticals To Limit Potential Carcinogenic Risk (FDA 2018b). We
note that FDA is not intending to apply ICH M7 (which is used to address impurities) to sunscreen active
ingredients.
32
Examples of such pathways could include endocrine function and signaling pathways related to growth and
development.
Proposed Order OTC000008
Page 23
Gestational and neonatal stages of development may be particularly sensitive to active
ingredients with hormonal activity (endocrine disruption). For this reason, these studies should
include assessments of endpoints such as vaginal patency, preputial separation, anogenital
distance, and nipple retention, which can be incorporated into traditional DART study designs to
assess potential hormonal effects on the developing offspring. Behavioral assessments (e.g.,
mating behavior) of offspring, which may detect neuroendocrine effects, should also be
performed (FDA 2015a).
3. Toxicokinetics (FDA 1995)
Animal toxicokinetic data should also be collected for sunscreen active ingredients, as
these data provide an important bridge between toxic levels seen in animal studies and any
potential human adverse events associated with systemic exposure to the sunscreen’s active
ingredient. Toxicokinetic measurements are usually obtained during the course of ongoing
nonclinical toxicity studies, such as carcinogenicity or DART studies, rather than through
separate studies.
iv. Postmarketing Safety Data
In addition to the active ingredient safety data already described, FDA’s GRASE
evaluation also takes into consideration publicly available information about serious adverse
drug experiences and known or expected adverse effects associated with commercially marketed
products that contain the active ingredient(s) under consideration.
v. Sunscreens Containing Nanomaterials
We note that FDA is not proposing to categorically classify sunscreen products
manufactured using nanotechnology (or containing nanomaterials) as GRASE or not GRASE
solely based on this characteristic. Nanotechnology is used to create, explore, or manipulate
materials measured in nanometers (nm) (billionths of a meter), and has applications in a wide
range of products, including OTC sunscreens. Such materials generally have dimensions
between approximately 1 and 100 nm (National Science and Technology Council Committee on
Technology 2014). Materials at such small sizes can have different chemical or physical
properties or biological effects compared to larger-scale counterparts, making possible a variety
of functional effects, and also potentially affecting the safety, effectiveness, or regulatory status
of FDA-regulated products.
FDA has not established regulatory definitions of nanotechnology, nanomaterial,
nanoscale, or other related terms. As described in FDA’s guidance for industry Considering
Whether an FDA-Regulated Product Involves the Application of Nanotechnology
(Nanotechnology Considerations Guidance) (FDA 2014a), at this time, when considering
whether an FDA-regulated product involves the application of nanotechnology, FDA asks:
Proposed Order OTC000008
Page 24
(1) Whether a material or end product is engineered to have at least one external
dimension, or an internal or surface structure, in the nanoscale range (approximately
1 nm to 100 nm).
In addition, because materials or end products can also exhibit related properties or phenomena
attributable to a dimension(s) outside the nanoscale range of approximately 1 nm to 100 nm that
are relevant to evaluations of safety, effectiveness, performance, quality, public health impact, or
regulatory status of products, we will also ask:
(2) Whether a material or end-product is engineered to exhibit properties or phenomena,
including physical or chemical properties or biological effects, that are attributable to
its dimension(s), even if these dimensions fall outside the nanoscale range, up to 1
micrometer (µm) ((1,000 nm).
We will apply these considerations broadly to all FDA-regulated products, including sunscreen
products. For the purpose of this proposed order, we use the term nanomaterial generally to
refer to materials falling within either point 1 or 2 above. The use of this term in this manner is
consistent with its use in FDA’s nanotechnology-related guidances, including FDA’s
Nanotechnology Considerations Guidance.
Nanomaterial forms of the active ingredients zinc oxide and titanium dioxide have been
used in marketed OTC sunscreens. In addition to nanomaterial forms of zinc oxide and titanium
dioxide, other nanomaterials are also reported to have been used, or promoted or studied for
possible use, in sunscreen products (Hayden et al. 2016).
As discussed in further detail in section VI.C.i, having examined the scientific
information in the record, including for nanomaterial forms of zinc oxide and titanium dioxide,
FDA is not now proposing conditions of use for these two active ingredients under the sunscreen
monograph that distinguish nanomaterials from other forms of these ingredients. As indicated
above, FDA also does not propose to categorically classify sunscreen products that are
manufactured using nanotechnology or contain nanomaterials as GRASE or not, solely on that
basis. Manufacturers of products containing nanomaterials marketed under the OTC sunscreen
monograph remain responsible for ensuring that the product satisfies all applicable legal
requirements. FDA encourages manufacturers of such products to consult with FDA to facilitate
a mutual understanding of specific scientific or regulatory issues relevant to their product.
FDA invites comment on the following topics:

Proposed Order OTC000008
Page 25
• Specific nanomaterials or types of nanomaterials that have been used or
proposed for use in OTC sunscreen products
• Concerns about sunscreen product safety, effectiveness, or quality associated
with the use of nanomaterials in OTC sunscreen products, with supporting
data
• Need for, and proposals of, specifications or limitations for particular
nanomaterials for use in OTC sunscreen products
• Any particular nanomaterials that you believe should not be permitted for use
in OTC sunscreen products, along with supporting scientific information
• FDA’s proposed regulatory approach and/or alternative regulatory approaches
to the use of nanomaterials in OTC sunscreen products
C. Existing Safety Data for Sunscreen Active Ingredients
In the remainder of this section, we discuss the existing data and data gaps for each of the
sunscreen monograph active ingredients and explain why we propose that these active
ingredients are GRASE or not GRASE for use in sunscreens.
i. Ingredients Proposed as GRASE for Use in Sunscreens
Based on our review of the publicly available data for these ingredients, we propose that
sunscreens containing zinc oxide, titanium dioxide, or both as active ingredients are GRASE
under section 201(p)(1) of the FD&C Act (and are not subject to section 503(b)(1) of the FD&C
Act), provided that they satisfy all the other conditions specified in a final sunscreen order. See
section 505G(b)(1)(A) of the FD&C Act.
1. Zinc Oxide
Our review of the scientific literature, submissions to the sunscreen monograph docket,
and adverse event reports submitted to FAERS has produced sufficient safety data on zinc oxide
to support a proposal that sunscreens containing up to 25 percent zinc oxide are GRASE under
section 201(p)(1) of the FD&C Act (and are not subject to section 503(b)(1)) provided that they
satisfy all the other conditions specified in a final sunscreen order. See section 505G(b)(1)(A) of
the FD&C Act. This proposal is based in significant part on the existing, substantial evidence
that zinc oxide (including particles on the nanoscale, i.e., approximately 1 to 100 nm) does not
penetrate into or through human skin to any great extent and, to the extent any de minimis
penetration occurs, does not result in adverse health effects, given the high levels of endogenous
zinc in the human system.
I. Background

Proposed Order OTC000008
Page 26
Zinc oxide is an inorganic, mineral compound. Because of its ability to reflect UVA
wavelengths, zinc oxide is frequently used in sunscreens to help establish broad spectrum
protection (Beasley and Meyer 2010).
While larger particles of zinc oxide used in sunscreens
(greater than approximately 100 nm) may impart an opaque, white color to the product, zinc
oxide is also manufactured in smaller particle sizes (less than approximately 100 nm) to reduce
this white/opaque appearance (Fairhurst and Mitchnick 1997; Mitchnick et al. 1999). In addition
to its use in sunscreens, zinc oxide is also used in non-sunscreen ointments, pastes, and lotions
for various skin disorders because of its protective, astringent, and antiseptic properties (Agren
1990b).
II. Discussion
Zinc oxide is insoluble in water and largely insoluble in biological fluids.
33
This
insolubility precludes the possibility of its systemic absorption from topical application of
sunscreen products beyond a de minimis amount, even if zinc oxide is included at its maximum
eligible concentration of 25 percent and regardless of the formulation of the product. The
available studies on the dermal penetration of zinc oxide, further discussed below, confirm that
its penetration—regardless of particle size—is primarily limited to the upper layers of the non-
living stratum corneum, with most penetration occurring only into skin folds and furrows or hair
follicles. These studies show that zinc oxide particles do not penetrate down into the viable dermis
to any significant extent. Any de minimis transdermal penetration that may occur does not result
in adverse health effects, because the tiny amount of zinc oxide particles that achieve
transdermal absorption, if any, would dissociate into zinc and oxygen ions, both of which are
naturally occurring elements in the human body (Samman et al. 2007). Zinc is the 14th most
common element in the human body and is essential for all living things; the average human
body contains about 2.0 to 2.5 grams of zinc, and normal dietary intake of zinc is about 15
milligrams (mg) per day (Samman et al. 2007; Plum et al. 2010). Homeostatic mechanisms in
the body regulate zinc’s absorption, distribution, cellular uptake, and excretion (Plum et al.
2010). Similarly, any oxygen absorbed through the skin is nonharmful, as oxygen is plentiful in
the human body and essential for life.
Our search of the literature on zinc oxide revealed four recent studies about zinc oxide’s
penetration into human skin, which confirm our expectations based on the physical properties of
this compound. The first two studies (conducted by Leite-Silva et al. and Darvin et al.)
examined the penetration of zinc oxide into the skin using multiphoton tomography (Leite-Silva
et al. 2013; Darvin et al. 2012). Both studies showed a lack of overall permeation of zinc oxide
beyond a few cell layers, except in the case of furrows and wrinkles (Leite-Silva et al. 2013;
Darvin et al. 2012). The second two studies—a pilot and subsequent full trial conducted by
Gulson et al.—evaluated the penetration of nanoscale zinc oxide into the skin and the
33
We note that nanoscale zinc oxide can be solubilized to a small extent in the presence of phosphate and lecithin at
pH’s that are achievable on the skin. Even under these conditions, however, the amount potentially absorbed is de
minimis and far lower than daily nutritional intake of zinc.

Proposed Order OTC000008
Page 27
bloodstream using a stable isotope tracing method (Gulson et al. 2010; Gulson et al. 2012).
Although the Gulson studies found that a minimal amount of topically applied zinc was
absorbed, the absorption observed was at levels that are orders of magnitude less than daily
nutritional intake and well below what would be of concern for a naturally occurring element in
the body subject to homeostatic mechanisms (Stefanidou et al. 2006). An additional porcine
study found (as discussed in our 2012 response to a citizen petition submitted by the
International Center for Technology Assessment and others (Docket No. FDA-2006-P-0213-
0003) (ICTA Petition Response)), that although sunburn caused by UVB rays increased the
penetration of zinc oxide into the non-living stratum corneum, there remained minimal
penetration of zinc oxide into the epidermal and dermal layers of the skin (Monteiro-Riviere et
al. 2011). Because topically applied zinc oxide particles do not enter systemic circulation to any
meaningful extent, we do not consider a MUsT program to be necessary to support the safety of
this ingredient.
In addition to the studies described above, we also located two studies evaluating the
clinical safety of topically applied zinc oxide in which zinc oxide (25 percent) was used as a
medicated occlusive dressing on the lower arms of healthy volunteers (Agren 1990a; Agren
1991). In these studies (which were designed to maximize potential absorption and identify any
resulting adverse events), even with the increased dermal or epidermal zinc levels resulting from
occlusion, there still were no adverse skin events. Our review of the available human dermal
safety studies on zinc oxide
34
also identified data showing that test material containing up to 25
percent zinc oxide did not induce human irritant, photoirritant, allergic, or photoallergic
reactions. No human pathological phototoxicity or significant human photosensitization reaction
indicative of skin irritation were noted either. The literature supporting the safety of skin
protectant drug products containing zinc oxide
35
reinforce these clinical safety findings. Our
review in this area is also consistent with the conclusion of the European Commission’s
Scientific Committee on Consumer Safety that the use of nanoscale zinc oxide in sunscreens at a
concentration of up to 25 percent does not pose a risk of adverse effects in humans after topical
application (European Commission 2012).
A very small number of rash and hypersensitivity reports for sunscreens containing zinc
oxide were located in FAERS. With a single exception, the sunscreens involved contained two
34
This literature included three clinical safety studies conducted by Hill Top Research, Inc. for Procter & Gamble
regarding (a) human sensitization (Study Reports 96-6635-76a and 96-6635-76b); (b) human
photoirritation/phototoxicity (Study Report 96-6634-6676); and (c) human photoallergenicity (Study Report 96-
6633-6376). See Citizen Petition submitted by Proctor & Gamble, June 24, 1997 (FDA-1978-N-0018-0639) and the
“Opinion concerning Zinc Oxide” drafted by the European Commission, Scientific Committee on Cosmetic
Products and Non-Food Products Intended for Consumers (SCCNFP), which included five summaries of human
clinical safety studies, all evaluating zinc oxide 25 percent (European Commission, 2012).
35
See, e.g., Beeckman et al. (Beeckman et al. 2009); 43 FR 34628 at 34641(August 4, 1978) (discussing use of zinc
oxide 1 percent to 25 percent as a skin protectant active ingredient: “Zinc oxide is widely recognized as a skin
protectant” and “No reports of topical toxicity were found in the literature” on zinc oxide).

Proposed Order OTC000008
Page 28
or more active ingredients, making it difficult to attribute causation to a specific active
ingredient. Unlike other sunscreen ingredients with a known hypersensitivity risk, we did not
identify any reports in FAERS or in the literature with features suggestive of a causative link,
such as skin test results positive for zinc oxide. In addition, there is an extremely large safety
database of zinc oxide use in other topical products, including for the treatment of diaper rash in
infants. This corroborates the negative results in human studies for irritation, photoirritation,
allergy, and photoallergy that support our proposed finding regarding the safety of sunscreens
containing this ingredient under the conditions proposed. Reports of non-hypersensitivity-
related clinical safety issues with zinc oxide were infrequent and not serious. For these reasons,
we do not consider additional clinical studies (including photosafety, irritation, or sensitization
studies) to be necessary for this ingredient.
Dermal carcinogenicity studies have not been conducted for zinc oxide. In general, as
discussed in section VI.B.iii.1, adequate tests for safety of an active ingredient for use in topical
products for chronic use (such as a sunscreen) would need to include dermal carcinogenicity
studies if the active ingredient reaches the viable layers of skin where it could have a biological
effect. Given the minimal penetration of zinc oxide below the non-living stratum corneum, there
is no plausible mechanism by which zinc oxide could have an effect on skin tumor development.
We are therefore proposing to find that zinc oxide is GRASE for use in sunscreens despite the
lack of dermal carcinogenicity studies studying this ingredient.
Based on the minimal systemic exposure resulting from dermally applied zinc oxide, in
particular when compared to endogenous zinc levels, we see no need for further nonclinical
studies to support the safety of sunscreens containing zinc oxide, including systemic
carcinogenicity studies, developmental and reproductive toxicity studies, or toxicokinetic
studies.
36
III. Conclusion
Our review of the available data from both animal and human studies and data on
physical properties such as solubility leads us to conclude that the transdermal absorption of zinc
oxide—regardless of particle size—from any topically applied sunscreen formulation is
extremely unlikely, and that any de minimis absorption that may occur would not result in
adverse health effects, given the high levels of endogenous zinc. The very low likelihood of any
systemic absorption of zinc oxide in turn indicates that the safety margin for zinc oxide is large;
accordingly, consistent with our approach to pediatric studies discussed in section VI.B.ii.3, we
do not consider pediatric studies to be needed for this ingredient. We propose to find that the
36
Our review of the available nonclinical safety literature on zinc oxide included references for a 90-day dermal
toxicity study, genotoxicity, and limited developmental and reproductive toxicity information. The review of this
literature suggests that genotoxicity findings for zinc oxide are mixed, and that there is minimal dermal toxicity in
rodents after 90 days (see Li et al. 2012; Ryu et al. 2014). Oral rat embryofetal toxicity studies showed some
adverse maternal and fetal effects, but only at very high doses (>200 mg/kg/day) significantly higher than what is at
issue here (Hong et al. 2014a; Hong et al. 2014b).

Proposed Order OTC000008
Page 29
currently available safety data provide sufficient evidence to demonstrate the minimal
absorption, low dermal irritation, low allergenic sensitization and photoallergenicity, and low
phototoxic potential of zinc oxide—regardless of particle size—up to 25 percent, and that these
data support a finding that zinc oxide up to 25 percent is GRASE for use in sunscreens under the
proposed conditions.
2. Titanium Dioxide
For similar reasons as for zinc oxide, we propose that sunscreens containing titanium
dioxide at up to 25 percent are GRASE under section 201(p)(1) of the FD&C Act (and are not
subject to section 503(b)(1) of the FD&C Act), provided they satisfy all the other conditions
specified in a final sunscreen order. See section 505G(b)(1)(A) of the FD&C Act. Our review
of information publicly available in the scientific literature, submissions to the sunscreen
monograph docket, and FAERS has produced sufficient information to support a proposal that a
sunscreen product containing up to 25 percent titanium dioxide would be GRASE under section
201(p)(1) of the FD&C Act (and not subject to section 503(b)(1) of the FD&C Act), provided it
complied with the other conditions in a final sunscreen order.
I. Background
Titanium dioxide is an inorganic mineral compound consisting of small, crystalline-
structured or amorphous particles. It is widely used as an excipient and is currently listed as an
inactive ingredient in more than 60 approved drug products (including topical, oral, and
inhalation products, among others) (FDA, n.d.). Titanium dioxide particles can be manufactured
to have a variety of different dimensions, shapes (such as spheres or rods), and crystal
polymorphs (such as anatase or rutile). Titanium dioxide (typically with particle dimensions
ranging from 200 to 300 nm) is manufactured as a white powder for use as a white color pigment
in pharmaceuticals. Manufacturers have also introduced processes that produce titanium dioxide
with particle dimensions ranging from 15 to 50 nm to reduce its white/opaque appearance.
Titanium dioxide particles used in sunscreens are also now often treated with chemical coatings
(such as silicones, metal oxides, or organic acids) that are bonded to the exterior surface of the
particles to, among other things, improve the aesthetic characteristics of the final formulation.
II. Discussion
Titanium dioxide is essentially insoluble in water and in biologic fluids (Gad and Wexler
2014). As with zinc oxide, this lack of solubility prevents the transdermal absorption of more
than a de minimis amount of titanium dioxide, regardless of either the concentration of titanium
dioxide or the formulation of the product (Pflucker and Hohenberg 2001). Further, unlike zinc
oxide, which, if dissolved, would dissociate into zinc and oxygen (Sandstead et al. 2015), the
chemical stability of titanium dioxide is such that it does not dissociate under the conditions that
exist in (or on) the human body (Gotman 2009).
Even if titanium dioxide were to dissociate into

Proposed Order OTC000008
Page 30
titanium and oxygen, titanium is unreactive in physiologic conditions, and (for this, among other,
reasons) is frequently used in medical devices and structures implanted in the human body
(Gotman 2009; Ipach et al. 2012).
The available studies on the transdermal absorption of titanium dioxide confirm that the
skin is an effective barrier to the penetration of titanium dioxide, regardless of particle size—
including those on the nanoscale (Sadrieh et al 2010; Kiss et al. 2008; Crosera et al. 2015). In
our 2012 response to the ICTA Petition mentioned earlier, we described the then available
information about the absorption of titanium dioxide nanomaterials and concluded that the
“currently available literature indicates that insoluble nanomaterials of titanium dioxide used in
sunscreens do not penetrate into or through human skin to produce adverse health effects when
applied topically” (ICTA Petition Response at 26). Our search of the available literature since
issuance of the ICTA petition response in advance of issuance of the 2019 proposed rule did not
reveal anything that changed this conclusion. Because topically applied titanium dioxide
particles do not enter systemic circulation to any meaningful extent, we do not consider a MUsT
program to be necessary for this ingredient.
Given the lack of transdermal absorption of titanium dioxide beyond a de minimis
amount and, as a result, the very low likelihood of any systemic effects, we also do not consider
additional nonclinical studies (including systemic carcinogenicity, developmental and
reproductive toxicity, or toxicokinetic) to be necessary to support the safety of this ingredient.
37
Because titanium dioxide penetration beyond the non-living stratum corneum and into the viable
layers of the skin is also minimal, as with zinc oxide, we do not consider dermal carcinogenicity
studies to be needed for titanium dioxide either.
The inability of more than an extremely minimal amount of titanium dioxide to reach
viable tissues that could have an immunologic reaction also prevents dermal irritation,
sensitization reactions, and photosafety issues for this ingredient. Our search of the available
literature on titanium dioxide identified nonclinical data reinforcing this, showing that dermal
toxicity after dermal application of titanium dioxide in rodents is minimal (Adachi et al. 2010;
Adachi et al. 2013; Unnithan et al. 2011a; Unnithan et al. 2011b). Accordingly, we do not
consider additional clinical photosafety, irritation, or sensitization studies to be necessary to
support the safety of this ingredient. We note that the available studies on titanium dioxide
evaluate products with titanium dioxide concentrations up to 10 percent. Given that the physical
properties of titanium dioxide both preclude its penetration into or through the human skin
regardless of concentration and make it unlikely that there would be dermal photosafety,
irritation, or sensitization associated with titanium dioxide exposure (and that there is no data to
37
We note that the available literature also includes data showing that oral administration of relatively high doses of
titanium dioxide did not produce adverse fetal effects in rats (see Warheit and Brown 2015)).

Proposed Order OTC000008
Page 31
suggest such photosafety, irritation, or sensitization would exist at higher concentrations), we
propose that titanium dioxide—regardless of particle size—is GRASE for use in sunscreens at
concentrations up to 25 percent, consistent with the level set in the 1999 Final Monograph.
In evaluating whether titanium dioxide is GRASE for use in sunscreen products, we have
considered published literature indicating that nanoscale titanium dioxide can exhibit
photocatalytic properties (Fujishima et al. 2000). The literature indicates that the crystalline
structure of titanium dioxide particles plays a role in this photocatalytic activity, and that the
anatase crystalline polymorph is associated with greater photocatalytic activity than the rutile
polymorph (Fujishima et al. 2000). The European Commission has established limitations on the
percentage of anatase crystalline polymorph in titanium dioxide to minimize photocatalytic
activity.
38
Coating titanium dioxide particles has also been shown to minimize photocatalytic
activity (and to limit particle clumping, which can have an impact on how products blend).
39
In theory, if photocatalytic activity occurred when sunscreen products containing
nanoscale titanium dioxide were exposed to light, it could result in the breakdown of other
sunscreen active ingredients in these products. We have no evidence, however, that this in fact
occurs in sunscreen products containing titanium dioxide or that there are any other negative
impacts resulting from such photocatalytic activity. Accordingly, its potential for photocatalytic
activity does not undermine our conclusion that titanium dioxide is GRASE for use in sunscreen
products. Nonetheless, we invite comment (including supporting data) on whether sunscreens
containing titanium dioxide are negatively impacted by the potential photocatalytic effects of that
ingredient and, if so, to what extent; and on additional regulatory conditions, if any, that are
necessary to address this potential issue.
We note, as well, that it is the responsibility of manufacturers to ensure that any inactive
ingredients used in a drug product marketed pursuant to the OTC Drug Review, including
coatings used to address photocatalytic activity or for other purposes, are safe and suitable for
their intended use (see 21 CFR 330.1(e)).
40
FDA encourages manufacturers to contact the
Agency regarding any specific coatings that they are considering for use in a topical sunscreen.
38
In a July 2013 opinion addressing the safe use of titanium dioxide in sunscreen products, the European
Commission’s Scientific Committee on Consumer Safety gave its opinion that titanium dioxide particles consisting,
among other things, of up to 5 percent anatase crystal “can be considered to not pose any risk of adverse effects in
humans after application on healthy, intact or sunburnt skin” (European Commission 2014). In 2016, this
physicochemical parameter was incorporated by the European Commission into its Regulation on Cosmetic
Products (Regulation (EC) No. 1223/2009 11/30/2009) permitting the use of titanium dioxide as a UV filter or as a
colorant in cosmetics. See Regulation (EC) No 1143/2016 July 13, 2016.
39
Id.
40
The substance of the general GRASE conditions established in 21 CFR 330.1 remains unchanged by the
enactment of section 505G, (see section 505G(k)(1) of the FD&C Act.

Proposed Order OTC000008
Page 32
III. Conclusion
Given the chemical properties of titanium dioxide as insoluble and unreactive under
physiologic conditions and the available studies showing that titanium dioxide does not penetrate
into the skin or enter into systemic circulation to any meaningful extent, we consider the
available safety data adequate to support a proposal that titanium dioxide is GRASE for use in
sunscreens. As with zinc oxide, our proposal rests in significant part on the data showing that
absorption of titanium dioxide into or through the skin is very unlikely and that any de minimis
absorption that could theoretically occur would not result in adverse health effects. As a result,
the safety margin here is large, and consistent with our approach to pediatric studies discussed in
section VI.B.ii.3, we therefore consider pediatric studies to be unnecessary for this ingredient.
ii. Ingredients Proposed as Not GRASE Due to Data Showing
Safety Issues
FDA’s review of the available safety data for PABA and trolamine salicylate have caused
us to conclude that the risks associated with use of these ingredients in sunscreen products
outweigh their benefits. Accordingly, we are proposing that sunscreens containing these two
ingredients are not GRASE under section 505G(b)(1)(C)(i) of the FD&C Act because the
evidence shows that sunscreens containing these ingredients are not GRASE.
1. Para-Aminobenzoic Acid
PABA use has decreased significantly in recent years because of, among other things, its
adverse effects on skin and its discoloring and staining effect on clothing. Our review of more
than 700 sunscreen brands sold in the United States (FDA 2019c)
indicates that PABA is in fact
no longer being marketed in the United States.
A search of the scientific literature, submissions to the sunscreen monograph docket, drug
approval documents from FDA and the European Medicines Agency, adverse event reports
submitted to FAERS, and FDA Advisory Committee meeting reports (among other sources) has
produced clinical safety data on PABA that supports a conclusion that a sunscreen containing
PABA would not be GRASE. The available clinical information includes significant numbers of
reports of allergic and photoallergic skin reactions to PABA, with rates of PABA-induced skin
reactions potentially 8 percent or higher (Balogh et al. 2011; Gao et al. 2014; Trevisi et al. 1994;
Victor et al. 2010).
An 8 percent incidence is a serious concern: by comparison, only 34
hypersensitivity reactions associated with sunscreen products have been identified in FAERS
since 1969.
41
41
Total sunscreen sales since 1969 are not readily available. However, in 2016, a total of 161,882,779 sunscreen
units were sold in the United States (Teplitz et al. 2018).
Proposed Order OTC000008
Page 33
Further, PABA has the ability to cause cross-sensitization to structurally similar aromatic
amines and nitro compounds (i.e., it can cause individuals exposed to it to develop sensitivity
reactions to similar compounds) (Willis 1976). The list of compounds at issue includes a variety
of widely used products, such as sulfonamide antibiotics (commonly used to treat a variety of
infections, from urinary tract infections to certain types of pneumonia), thiazide diuretics (the
number one recommended treatment for hypertension for certain communities), certain local
anesthetics (such as benzocaine and procaine), and dyes (including para-phenylenediamine (a
hair dye) and aniline dyes (used in medical products)) (Fisher 1992; Mackie and Mackie 1999;
Pathak 1982). Cross-sensitization to these products is a serious concern, as widespread PABA
use could result in a significant increase in cross-reactivity with these agents and the incidence of
allergic and photoallergic dermatitis, some of which are likely to be severe.
These safety issues alone are reason enough to find PABA not GRASE for use in
sunscreens. In addition, however, data obtained from the urine samples of human subjects
receiving topical PABA application shows that PABA also penetrates the skin and enters
systemic circulation (Wang et al. 2007). Because full MUsT studies for PABA have not been
done, it is unclear to what degree such transdermal absorption takes place. However, one article
in the published literature suggests that there is an association between autoimmune disorder and
PABA use (Mackie and Mackie 1999), and we found one report each of hepatotoxicity (Borum
et al. 1991) and chronic interstitial nephritis (Alexopoulos et al. 1993) after oral PABA
administration. Although it is difficult to determine causality on the basis of such single reports,
if a MUsT program were to show absorption of PABA, these reports could represent an
additional safety concern.
In addition, genotoxicity findings with PABA use have been largely negative in the
absence of UV irradiation. Adequate assessments of the dermal carcinogenicity potential of
PABA are unavailable, as are DART studies. If a MUsT program were to show absorption of
PABA, therefore, necessary studies would include dermal and systemic carcinogenicity studies,
DART studies, and toxicokinetic studies. However, given that the above-described safety
concerns associated with PABA are significant enough for FDA to determine that the evidence
shows that sunscreens containing PABA are not GRASE, conducting such testing is neither
appropriate nor ethical. We propose that PABA is not GRASE for use in sunscreens under
section 505G(b)(1)(C)(i) of the FD&C Act.
2. Trolamine Salicylate
We also propose that trolamine salicylate is not GRASE for use in sunscreens under
section 505G(b)(1)(C)(i) of the FD&C Act. As described in further detail below, there are
significant safety concerns associated with the use of trolamine salicylate in sunscreen products.
We propose that these concerns are sufficient to support a conclusion that the evidence shows

Proposed Order OTC000008
Page 34
that a sunscreen containing trolamine salicylate would not be GRASE. We note that, as with
PABA, our review of more than 700 sunscreen brands sold in the United States suggests that
trolamine salicylate is no longer being marketed in sunscreens sold in the United States (FDA
2019c).
I. Background
Trolamine salicylate is comprised of trolamine and salicylic acid. Salicylic acid is a non-
steroidal anti-inflammatory drug (NSAID); it is the active moiety in aspirin and has been widely
used as an analgesic (i.e., pain relieving), anti-pyretic (i.e., fever reducing), and anti-
inflammatory agent. In addition to these properties, salicylic acid inhibits platelet aggregation,
which in turn inhibits blood coagulation. For this reason, some salicylic acid-containing
products (such as aspirin) are used by consumers to help reduce cardiovascular adverse events,
including myocardial infarction, stent thrombosis, and transient ischemic attacks.
Trolamine salicylate was included in the 1999 Final Monograph for sunscreens at a
concentration of up to 12 percent. In the tentative final monograph
42
for OTC external analgesic
drug products, it was proposed as not GRASE because additional data were needed to support a
GRASE finding (“External Analgesic Drug Products for Over-the-Counter Human Use;
Tentative Final Monograph,” 48 FR 5852 at 5855 (February 8, 1983)) (External Analgesic
TFM). The mechanisms of action for trolamine salicylate for these two drug categories are very
different; to be effective as an external analgesic, trolamine salicylate must penetrate the skin and
reach the relevant sites of action. The available evidence clearly establishes that trolamine
salicylate is transdermally absorbed (Rose and Wiemer 1983; Madan and Levitt 2014). To be
effective as a sunscreen, however, trolamine salicylate must be present on the surface of the skin
so that it can reflect, scatter, or absorb UV radiation.
The directions for use for the two product categories differ significantly as well. The
current requirements for sunscreen labeling include directions that the product should be applied
to all skin exposed to the sun, that it should be used “regularly” to decrease the risk of skin
cancer and early skin aging,
43
and that it should be reapplied at least every 2 hours (see Deemed
Final Order at § M020.50(e)). In contrast, currently marketed external analgesic products
containing trolamine salicylate include directions for use stating that they should be applied to
“affected areas,” that they should be reapplied no more than three to four times a day, and that
use should be discontinued after 7 days.
44
42
Under the rulemaking system for the OTC Drug Review that preceded the passage of the CARES Act, the Agency
would publish a proposed rule proposing conditions under which OTC drugs in the therapeutic class being
considered were GRASE and not misbranded. These proposed rules were called tentative final monographs.
43
This direction applies to sunscreens with an SPF of 15 or greater that are also broad spectrum.
44
Based on an evaluation of product labeling available at https://labels.fda.gov
(accessed May 27, 2021). See also External Analgesic TFM.

Proposed Order OTC000008
Page 35
II. Significant safety concerns associated with use
of trolamine salicylate as a sunscreen
FDA is concerned that use of trolamine salicylate as an active ingredient in sunscreens
could cause serious detrimental health effects due to the anti-coagulation effects of salicylic acid.
FDA located two case reports of serious coagulation-related adverse events associated with
liberal dermal application of trolamine salicylate. The first case involved a surgical patient who
experienced coagulopathy (impairment of the blood’s ability to coagulate) at surgical sites in
connection with use of topical trolamine salicylate (Rose and Wiemer 1983).
Although the
patient discontinued aspirin use 2 weeks before surgery per her doctor’s instructions, she was
unaware that use of a topical cream containing trolamine salicylate should have been stopped as
well, and continued liberal application of the product to her knees for arthritis pain in the period
leading up to her surgery. Four hours after surgery, the patient returned to the operating room
bleeding profusely from all surfaces that had been operated on and experiencing massive
bilateral hematomas. She lost more than 900 mL of blood.
In the second case, a patient taking warfarin (an anticoagulant) for atrial fibrillation and
stroke prevention experienced a considerable increase in prothrombin time (i.e., the time it takes
for blood to coagulate) after liberal application of trolamine salicylate to his neck and shoulders
for pain relief (Littleton 1990). The patient’s prothrombin time had previously been in the
therapeutic range of 1.3 to 1.5 times the control, but increased to 2.5 times the control during
trolamine salicylate use. When trolamine salicylate use was discontinued, the patient’s
prothrombin time returned to 1.3 times the control.
FDA is also concerned that sunscreens containing trolamine salicylate could lead to other
adverse effects associated with salicylic acid exposure. These include gastrointestinal distress
and hemorrhage, ototoxic effects (i.e., impacts on hearing), hypersensitivity reactions, asthma
exacerbations, acid-base imbalance, salt and water retention, liver injury, and Reye’s Syndrome
(in children).
At high doses, acute salicylate toxicity (salicylism) may occur. Early symptoms
of salicylism include tinnitus, vertigo, nausea, vomiting, and diarrhea; subsequent symptoms
suggesting a more severe intoxication include altered mental status (ranging from agitation to
lethargy), hyperpyrexia, noncardiac pulmonary edema, and coma.
45
If trolamine salicylate were to be applied to all skin exposed to the sun and reapplied
every 2 hours as directed in sunscreen labeling, the potential for transdermal absorption and
systemic availability of substantial amounts of salicylic acid raises significant concerns about the
potential for increased occurrence of the above-described adverse events. This is a particular
45
The symptoms associated with both acute and chronic salicylate toxicity are well established. Descriptions are
available from many sources, including: National Library of Medicine’s Toxicology Data Network (ToxNet),
“Salicylic Acid,” September 2008, available at https://toxnet.nlm.nih.gov/cgi-
bin/sis/search/a?dbs+hsdb:@term+@DOCNO+672 (accessed March 27, 2018). A comprehensive summary of
salicylism can also be found in
§ M013.

Proposed Order OTC000008
Page 36
concern given the widespread use of other OTC NSAID products with anti-inflammatory,
analgesic, or anti-pyretic effects, which, combined with the use of sunscreens containing
trolamine salicylate, may raise the anti-platelet effects experienced by consumers to problematic
levels. Concerns relating to transdermal absorption may be especially acute for children, who
have a higher surface-area-to-body-weight ratio than adults. FDA proposes that the above-
described safety concerns are enough, by themselves, to support a finding that trolamine
salicylate is not GRASE for use in sunscreens.
III. Data gaps
In addition, there are several categories of data about trolamine salicylate that FDA
expects would be necessary to support a positive GRASE determination for its use in sunscreen
products that are currently missing from the public record. For example, there is insufficient
clinical dermal sensitization, irritation, and photosafety data for trolamine salicylate. Although
the transdermal absorption of trolamine salicylate is well established, the record currently lacks a
MUsT program that would allow us to evaluate the extent of exposure to this ingredient when it
is used as a sunscreen. Such data is important because it would allow FDA to interpret systemic
toxicity findings in animal toxicology studies in the context of the amount likely to be absorbed
from sunscreen use. Given the FDA recommendation that a MUsT program for sunscreen use
include application to a majority (75 percent at a minimum) of the body surface of each test
subject, the above described safety concerns (including the potential for salicylism associated
with exposure to high doses of trolamine salicylate) would raise significant ethical concerns
about the conduct of a MUsT program in these circumstances. Were it possible to ethically
conduct a MUsT for this ingredient, and if such a MUsT showed significant transdermal
absorption of trolamine salicylate, this would raise questions about whether enough of this
ingredient remains present on the surface of the skin for it to function effectively as a sunscreen.
As we noted in section VI.B.ii.2, such considerations ultimately weigh into the risk-benefit
calculus FDA uses to determine whether an active ingredient would be GRASE for use in
sunscreens.
Although we have data addressing the toxicology profile of salicylate, adequately
detailed nonclinical DART studies for trolamine and toxicokinetic data to interpret DART
studies were also not found in the public record. Adequate DART information, if it were
available, might reveal additional data needs (for example, to address any potential hormonal
effects that may be identified). Dermal carcinogenicity data are available from the National
Toxicology Program for trolamine in acetone and trolamine alone (applied neat).
46
In the
absence of toxicokinetic data to interpret existing carcinogenicity studies, we cannot determine
46
In mice, liver tumors were identified, providing evidence of systemic absorption of trolamine, but the suspected
mechanism of action is likely not relevant to humans (National Toxicology Program 2004; National Toxicology
Program 1999). A causal link between the proposed mechanism and tumor formation in mice is lacking.
Proposed Order OTC000008
Page 37
how the exposure in the animal studies relates to human exposure to trolamine from the use of
trolamine salicylate as a sunscreen active ingredient.
IV. Conclusion
For the reasons described above, FDA proposes that trolamine salicylate is not GRASE
for use in sunscreens. The safety concerns associated with the use of trolamine salicylate as an
active ingredient in sunscreens are significant enough to support a determination that the
evidence shows that sunscreens containing trolamine salicylate are not GRASE pursuant to
section 505G(b)(1)(C)(i) of the FD&C Act. In particular, the potential for transdermal
absorption and systemic availability of substantial amounts of salicylic acid in connection with
the exposure resulting from the use of trolamine salicylate in sunscreens raises concerns about
increased occurrence of the above-described serious adverse events (including salicylism and
serious coagulation-related issues). The record also contains several significant data gaps that
would need to be addressed to support a positive GRASE determination for trolamine salicylate.
Given the safety concerns described above, however, conducting the clinical absorption testing
recommended to address these gaps for use as a sunscreen raises ethical concerns.
iii. Ingredients Proposed as Not GRASE Due to Insufficient Data
The public record does not contain sufficient data to support a positive GRASE
determination for cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate,
octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, or avobenzone at this time.
Accordingly, we propose that sunscreens containing these ingredients are not GRASE under
section 505G(b)(1)(C)(ii) of the FD&C Act because the evidence is inadequate to show that
sunscreens containing these ingredients are GRASE. In the sections that follow, we discuss our
review of the available safety evidence for these ingredients and identify the existing data gaps.
1. Ingredients for Which the Record Contains Significant Data Gaps:
Cinoxate, Dioxybenzone, Ensulizole, Homosalate, Meradimate,
Octinoxate, Octisalate, Octocrylene, Padimate O, and
Sulisobenzone
The most significant gaps in the administrative record exist for the following active
ingredients: cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate,
octocrylene, padimate O, and sulisobenzone. We expect that data from all the types of studies
described in section VI.B will need to be submitted to support general recognition of safety and
effectiveness for each of these ingredients.
At the time of FDA’s 2019 Proposed Rule, only three of these active ingredients
(homosalate (Benson et al. 2005)), octinoxate (Benson et al. 2005; Janjua et al. 2008; Janjua et
al. 2004; Sarveiva and Benson 2004), and octisalate (Benson et al. 2005), for example, appeared

Proposed Order OTC000008
Page 38
to have been evaluated in human absorption studies, and most of the available absorption studies
for these three ingredients had significant limitations. For example, the studies used a limited
number of subjects or were based on only a single application of the sunscreen active ingredient
to a limited area of the body. Even with this limited sunscreen exposure, some of these studies
showed systemic availability of the active ingredient (octinoxate (Janjua et al. 2004; Sarveiva et
al. 2004)). As noted in section IV above, in the time since the 2019 Proposed Rule, FDA has
conducted two pilot studies on the absorption of certain sunscreen ingredients
47
(formulated in
marketed sunscreen products) in order to model the conduct and feasibility of MUsT studies for
sunscreen ingredients. Both studies confirmed our understanding that these ingredients were
absorbed through the skin and into the bloodstream. To date, however, none of the 10
ingredients discussed in this section have been studied in an adequate, sufficiently powered
MUsT program that would determine the amount of systemic exposure to the active ingredients
under conditions of maximal use.
We note that an earlier publication examining the relationship between melting point,
molecular weight, and the transdermal delivery rates of the active ingredients in approved drug
products shows that products containing active ingredients with melting points and molecular
weights similar to many of these 10 sunscreen active ingredients are among those successfully
delivered transdermally—and therefore available systemically (Pastore et al. 2015). This
potential for transdermal absorption of and systemic exposure to these sunscreen ingredients was
confirmed in the two more recent absorption studies described above. Such systemic exposure is
a concern because the available data are inadequate to determine either the level of systemic
exposure to these active ingredients or the potential unintended consequences of such exposure.
Given the lack of chronic exposure toxicology data for these 10 ingredients—which makes an
evaluation of the dermal and systemic effects of chronic use impossible—this is especially
concerning. A number of these active ingredients have also shown hormonal effects in
mammalian assays (homosalate (Gomez et al. 2005; Schreurs et al. 2002; Schlumpf et al. 2001;
European Comission, n.d.; Schreurs et al. 2005; Ma et al. 2003; Krause et al. 2012)) and
padimate O (64 FR 27666 at 27671) and in in vitro and in vivo assays (homosalate (Gomez et al.
2005; Schreurs et al. 2002; Schlumpf et al. 2001; European Comission, n.d.; Schreurs et al. 2005;
Ma et al. 2003; Krause et al. 2012),
octinoxate (Szwarcfarb et al. 2008; Seidlova-Wuttke et al.
2006),
and octocrylene (Freitas et al. 2015). Although these findings are only preliminary, we
do not have adequate DART studies to enable us to assess the impact of these potential hormonal
effects on development and reproduction.
In addition, several of these 10 ingredients (homosalate (Benson et al. 2005; Sarveiva and
Benson 2004), octinoxate (Benson et a. 2005; Montenegro and Puglisi 2013;
Golmohammadzadeh et al. 2008; Montenegro et al. 2008 et al. 2008; Gupta et al. 1999; Hayden
47
Avobenzone, oxybenzone, octocrylene, homosalate, octisalate, octinoxate, and ecamsule.

Proposed Order OTC000008
Page 39
et al. 2005; Treffel and Gabard 1996),
octisalate (Benson et al. 2005; Sarveiva and Benson 2004;
Treffel and Gabard 1996; Jiang et al. 1997; Walters et al. 1998; Walters et al. 1997; Santos et al.
2012),
octocrylene (Freitas et al. 2015; Potard et al. 2000), padimate O (Hayden et al. 2005), and
sulisobenzone (Kurul and Hekimoglu 2001; Brinon et al. 1999)) have been studied in dermal
penetration studies, which also show (in general, with the exception of homosalate
48
) that these
ingredients permeate into the epidermis and/or dermis. The studies show that there are several
factors (including vehicle composition and the presence of other active ingredients) that can
influence, and potentially increase, the permeation and/or penetration of these ingredients.
Because the record does not currently contain sufficient data to support their safety, we
are proposing that sunscreens containing cinoxate, dioxybenzone, ensulizole, homosalate,
meradimate, octinoxate, octisalate, octocrylene, padimate O, and sulisobenzone are not GRASE
under section 505G(b)(1)(C)(ii) of the FD&C Act because the evidence is inadequate to show
that they are GRASE. As previously noted, we expect that data from all the types of studies
described in section VI.B will be needed to support general recognition of safety and
effectiveness for these ingredients for use in sunscreens.
2. Ingredients for Which the Record Contains Fewer Data Gaps:
Oxybenzone and Avobenzone
While the record does not currently contain sufficient data to support positive GRASE
findings for oxybenzone and avobenzone, we have significantly more data for these two
ingredients than for the ingredients discussed in the preceding section. To help facilitate
submission of the remaining data, we describe the data gaps for these two ingredients in greater
detail below.
I. Oxybenzone
Although we located substantially more data on oxybenzone than on the ingredients
discussed in section VI.C.iii.1, our review of the scientific literature, submissions to the
sunscreen monograph docket, and postmarket safety data publicly available through FAERS
revealed significant gaps in the data we expect to be necessary to support a positive GRASE
finding for use of oxybenzone at a concentration of up to 6 percent in sunscreen products. The
available literature includes studies indicating that oxybenzone is absorbed through the skin and
can lead to significant systemic exposure, as well as data showing the presence of oxybenzone in
human breast milk, amniotic fluid, urine, and blood plasma. The significant systemic availability
of oxybenzone (and, as discussed further below, the lack of data evaluating the full extent of its
absorption potential) is a concern, among other reasons, because of questions raised in the
published literature regarding the potential for endocrine activity with systemic oxybenzone
exposure. Accordingly, we expect that a positive GRASE finding for oxybenzone-containing
sunscreens would require, among other things, both an adequate MUsT program showing the
48
We note that the absorption studies FDA conducted did show that homosalate was absorbed into the body.
Proposed Order OTC000008
Page 40
degree of oxybenzone absorption under maximal usage conditions and DART studies that fully
investigate its potential endocrine-disrupting effects. We found neither in the existing record.
The record also lacks systemic and dermal carcinogenicity studies for oxybenzone; these
(and toxicokinetic data) should also be provided to support a positive GRASE finding for this
ingredient. Finally, the available literature also raises questions about the safety of use of
oxybenzone-containing sunscreens in young children because of the potential for higher
absorption and bioaccumulation of oxybenzone in this population. As discussed in further detail
in the sections that follow, we invite input and comment on appropriate studies and/or age
restrictions to address these pediatric issues.
i. Background
Unlike zinc oxide and titanium dioxide, both of which are inorganic (or physical) UV
filters consisting of metal oxides that primarily reflect or scatter UV radiation, oxybenzone is an
organic (or chemical) filter, which absorbs UV radiation. It belongs to a class of aromatic
ketones known as benzophenones and has a UV absorption profile covering both UVA and UVB
wavelengths (Burnett and Wang 2011). Because of its superior UVA coverage, oxybenzone was
increasingly used through the early 1990s and ultimately replaced PABA in sunscreen products
(Palm and O’Donoghue 2007). Use of oxybenzone in sunscreens increased when “PABA-free”
sunscreens were introduced into the market because of recognition that PABA and its esters
induced contact and photocontact allergic reactions (Palm and O’Donoghue 2007). As discussed
below, however, evidence shows that oxybenzone also has contact allergenic and photoallergenic
potential (European Commission 2008). In addition to its use as a sunscreen active ingredient,
oxybenzone is used in, among other things, perfumes, lipsticks, hair sprays, and conditioners as a
photostabilizer and/or fragrance enhancer (Environmental Working Group 2008; Kadry et al.
1995).
ii. Data showing transdermal absorption
and significant systemic availability of
oxybenzone
Data that have become available since publication of the 1999 Final Monograph suggest
that the transdermal absorption of oxybenzone is high (Janjua et al. 2008; Calafat et al. 2008;
Gustavsson et al. 2002). One study involving sampling of plasma and urine following topical
application of an oxybenzone-containing formulation showed absorption and significant
systemic availability of oxybenzone (Janjua et al. 2008). In this study, 15 men and 17 women
were dosed once daily, applying a 10 percent oxybenzone cream formulation to approximately
90 percent of the body’s surface area for 4 days. The figures below illustrate the plasma and
urine levels observed.
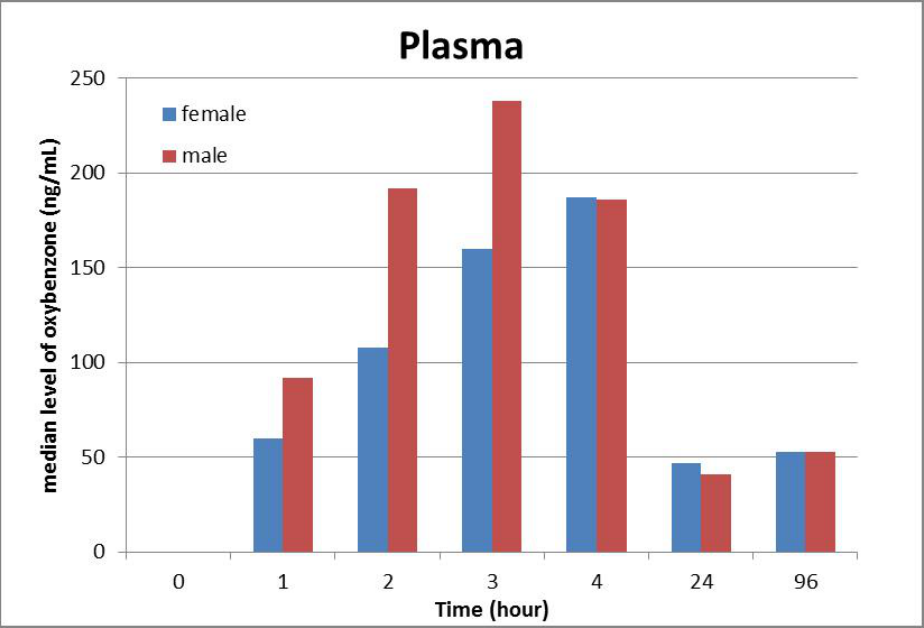
Proposed Order F000001
Page 39
Figure 1.--Plasma Concentration of Oxybenzone with Daily Topical Application
Source: Data adapted from Janjua et al., 2008 (Janjua et al. 2008).
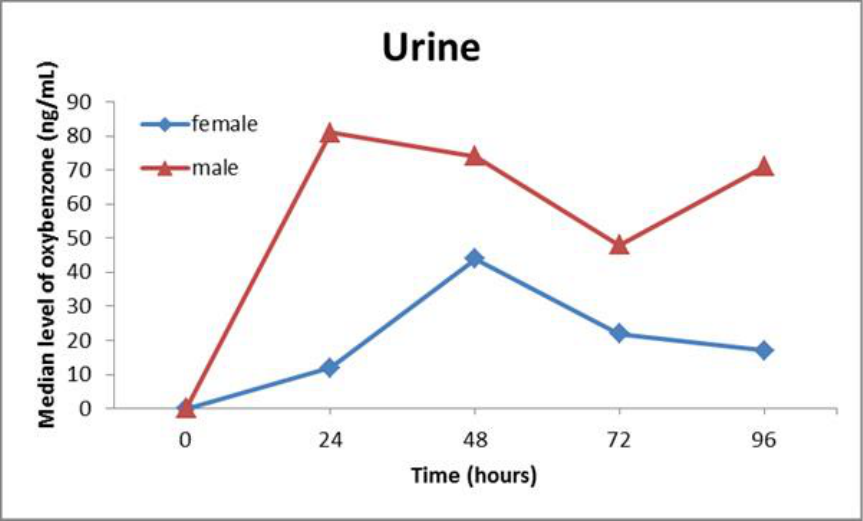
Proposed Order OTC000008
Page 40
Figure 2.--Urine Concentration of Oxybenzone with Daily Topical Application
Source: Data adapted from Janjua et al. (2008).
Although this study provides important information about the significant absorption
potential of oxybenzone, it does not obviate the need for a MUsT program. Among other things,
once-daily application may result in substantially lower systemic exposure than application at
least every 2 hours (as sunscreen labeling directs). This difference in application frequency is a
particular concern given that the data show oxybenzone levels would still be increasing at the
time of reapplication if a 2-hour application window were observed. Additionally, the cream
formulation used in the study was not formulated as a sunscreen product and may have contained
ingredients not typically used in sunscreen formulations, and/or lacked other ingredients
typically present. Because the formulation can have an impact on absorption, the absorption
results produced by the study may not reflect absorption levels that would result from actual use
of oxybenzone-containing sunscreen products.
Another study, which evaluated the transdermal absorption of a marketed sunscreen
containing 4 percent oxybenzone in 16 women and 9 men, showed prolonged systemic
availability of oxybenzone following topical exposure (Gustavsson et al. 2002). In this study,
which was designed to evaluate the effects of UV radiation on oxybenzone absorption, the
sunscreen was applied to study subjects twice daily for 5 days. Although the study concluded
that UV exposure did not significantly affect the urinary excretion of oxybenzone, it provided
further evidence of the systemic availability of oxybenzone following topical application and
showed that renal excretion of oxybenzone continued for 5 days after the last application of the
sunscreen. Although the use of a commercial sunscreen formulation, and twice- rather than

Proposed Order OTC000008
Page 41
once-daily sunscreen application are improvements over the formulation and application
frequency used in the previous study, twice-daily application remains insufficient to approximate
the recommended application frequency of sunscreen products in real-world use. Furthermore,
because the study used a sunscreen with 4 percent rather than the full 6 percent concentration of
oxybenzone eligible for the sunscreen monograph, its results may not fully reflect the absorption
that would result from use of a 6 percent oxybenzone-containing product. To properly
characterize the potential for absorption of oxybenzone in sunscreen products and to determine a
margin of safety for use of oxybenzone at up to 6 percent in sunscreen products, we expect that a
MUsT program will be needed. As noted above, while oxybenzone’s absorption was evaluated in
the pilot absorption studies conducted by FDA referenced earlier, these studies were not
sufficiently powered to eliminate the need for a full MUsT program for this ingredient.
iii. Inadequate data on oxybenzone’s
developmental and reproductive toxicity
The significant systemic availability of oxybenzone following topical application and the
lack of data fully characterizing its absorption levels are concerns, among other reasons, because
of literature suggesting that oxybenzone may have endocrine activity (see, e.g., Schlumpf et al.
2001; Krause et al. 2012; Schlumpf et al. 2004). Dermal exposure to oxybenzone (in acetone) in
rats and mice and oral feeding of oxybenzone to rats and mice resulted in reduced sperm density
in males in 13-week general toxicity studies conducted by the National Toxicology Program
(NTP) (French 1992). In female rats and mice, increased estrous cycle length was observed in
13-week oral feeding studies.
49
Importantly, the actual effects of oxybenzone on female fertility
were not evaluated. In a preliminary dose range-finding pre- and postnatal development study in
rats, findings in male offspring indicated that cells in the testes undergoing programmed cell
death were increased in all oxybenzone-exposed animals and that numbers of spermatocytes in
the testes were markedly reduced after oral feeding at oxybenzone (Nakamura et al. 2015).
Although these findings are notable, they are all derived from dermal studies with oxybenzone in
acetone and oral feeding studies of oxybenzone; these methods of exposure could potentially
lead to higher levels of systemic exposure to oxybenzone than with sunscreen use. Accordingly,
a MUsT and toxicokinetic data are needed to determine the relevance of these findings to human
use of oxybenzone as a sunscreen active ingredient.
In humans, the endocrine effects of oxybenzone have been studied with inconclusive
results (see, e.g., Janjua et al. 2004; Janjua et al. 2007; Schiffer et al. 2014). In biomonitoring
studies of pregnant and lactating women, oxybenzone has been detected in breast milk, amniotic
fluid, and urine samples (Janjua et al. 2004; Janjua et al. 2007; Schiffer et al. 2014). High levels
of oxybenzone in the urine of mothers have been associated with: (1) decreased birth weight in
girls and (2) increased birth weight and head circumference in boys, both of which can be
indications of endocrine effects (Janjua et al. 2004). This association is particularly concerning
49
These changes could potentially be addressed with historical control data (Schlumpf et al. 2001).

Proposed Order OTC000008
Page 42
given the widespread exposure of the U.S. population to oxybenzone. Estimates suggest that
oxybenzone (from all sources) is present in the urine of 97 percent of the U.S. population, and
that oxybenzone concentrations are higher in women than in men (possibly because women are
more likely to use sunscreen and other personal care products containing oxybenzone, leading to
greater cumulative exposure) (Janjua et al. 2004; Callafat et al. 2008).
Because current data suggest that oxybenzone may affect the human endocrine system,
FDA believes that a positive GRASE determination for oxybenzone would require that its
potential toxicities have been fully explored, including through DART studies (fertility and early
embryonic studies in rodents, embryofetal development studies in rodent and nonrodent species,
and pre- and postnatal development studies in rodents). In addition, as noted below,
toxicokinetic data are needed to interpret these studies. We note that, if the results of DART
studies do not resolve the concerns raised in the literature relating to potential endocrine
disruption, it may still be possible to resolve these concerns through additional testing.
50
In
addition, because of the potential risk posed by metabolites of oxybenzone (existing reports
suggest that some oxybenzone metabolites are more hormonally active than the parent drug
(Burnett and Wang 2011)), we recommend that the analytical method used in the MUsT program
be validated for both the parent and the metabolites of interest (Calafat et al. 2008) to support a
positive GRASE finding for this ingredient. The results from the metabolite study will inform
whether additional nonclinical studies assessing oxybenzone’s metabolites should be conducted
to support its safety.
We note that in the time since the publication of the 2019 Proposed Rule, NTP and
NCTR have made progress on some DART studies on oxybenzone, although at the time of this
Proposed Order, many of the planned peer reviewed final study reports have not been published
(National Center for Toxicological Research, n.d.; National Toxicology Program, n.d.). In
addition, neither NCTR nor NTP plan to conduct an embryofetal development assessment in a
nonrodent species. Because the full battery of DART studies is not available, DART studies are
listed below as a data gap.
iv. Inadequate carcinogenicity and
toxicokinetic data for oxybenzone
High population exposure to oxybenzone, coupled with a lack of carcinogenicity testing
for this ingredient, caused the National Cancer Institute to nominate oxybenzone for toxicology
testing in 1979 (National Toxicology Program 1979). Since publication of the 2019 Proposed
Rule, NTP concluded 2-year oral (dosed feed) carcinogenicity studies in rats and mice. FDA is
reviewing these study reports (National Toxicology Program, n.d.). Animal toxicokinetic data
and clinical exposure data, however, are not available to interpret how the studies relate to the
50
For examples of the type of studies that could be explored at that juncture, see FDA 2015b.

Proposed Order OTC000008
Page 43
safety of oxybenzone when used as a sunscreen, nor does NTP plan to conduct these studies. In
addition, no reports of dermal carcinogenicity studies for oxybenzone are available, and NTP
does not plan to conduct a dermal carcinogenicity assessment. To support a positive GRASE
finding for oxybenzone, carcinogenicity data from well-conducted dermal and systemic
carcinogenicity studies are needed. However, it is possible that the NTP oral carcinogenicity
studies will fill the data gap for systemic carcinogenicity. Toxicokinetic data in rodents (oral and
dermal) and rabbits (oral) are also needed to interpret the DART and carcinogenicity studies.
Toxicokinetic data could be obtained from either stand-alone studies or as part of DART and
carcinogenicity studies.
Our search of the available literature also revealed information suggesting that
oxybenzone may generate reactive oxygen species (ROS)
51
in the presence of UV light, but that
this issue, and the harms associated with it, have not been fully explored (Hanson et al. 2006).
We invite comment and input on the extent to which ROS generation is a concern for sunscreens
containing oxybenzone and whether additional data on this topic are needed.
v. Dermal safety of oxybenzone
The available data indicate that oxybenzone (at concentrations up to 6 percent) has a
favorable safety profile with respect to irritation and sensitization potential. For example, the
North American Contact Dermatitis Group conducted an analysis of patients who were patch
tested for allergies between 2001 and 2010 (see, e.g., Warshaw et al. 2013). From 2001 to 2008,
oxybenzone was tested at 3 percent; from 2009 to 2010, the concentration used for the test was
increased to 10 percent. Of the 23,908 patients patch tested, only 82 patients (0.34 percent) had
positive test patch results with oxybenzone. In addition, a search of FAERS for case reports of
hypersensitivity reactions to oxybenzone-containing sunscreen products resulted in only 31 cases
(4 with anaphylaxis) between 1988 and 2011. Because sufficient data exist to make a
determination, we do not consider additional dermal irritation or sensitization studies to be
necessary to support a positive GRASE finding for oxybenzone up to 6 percent. As is customary
in clinical trials, however, we recommend that dermal safety data for oxybenzone be collected
during MUsT studies.
Nevertheless, the overwhelming majority of results from available studies (see, e.g.,
Warshaw et al. 2013; Ang et al. 1998; Bruynzell et al. 2004; Bryden et al. 2006; Dromgoole and
Maibach 1990; European Multicentre Photopatch Test Study (EMCPPTS) Taskforce 2012;
Heurung et al. 2014a; Heurung et al. 2014b; Journe et al. 1999; Rodriguez et al. 2006; Schauder
and Ippen 1997; Valbuena et al. 2016 ) addressing allergic contact dermatitis for oxybenzone
show that oxybenzone is an allergen for persons with preexisting skin conditions. Because the
51
Reactive oxygen species are “a type of unstable molecule that contains oxygen and that easily reacts with other
molecules in a cell. A buildup of ROS in cells may cause damage to DNA, RNA, and proteins, and may cause cell
death.” https://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=687227
(accessed May 27, 2021)
Proposed Order OTC000008
Page 44
evidence establishing oxybenzone as a photoallergen in individuals with photosensitivity is clear,
no further dermal photosafety studies to characterize this risk are needed. However, if we were
to receive adequate data to support a positive GRASE finding for oxybenzone, we would
consider requiring labeling language to address the risk of allergic reactions associated with
oxybenzone use. We invite comment on whether such labeling should be required for
sunscreens containing oxybenzone and, if so, what that labeling should entail.
vi. Safety questions regarding use of
oxybenzone in pediatric populations
Sunscreens are currently labeled for use in children as young as 6 months old. The
available literature, however, includes several publications that raise concerns about the use of
sunscreens containing oxybenzone in young children. Among these publications is a 2006 report
from the Swedish Research Council noting that children under the age of 2 years old have not
fully developed the enzymes believed to metabolize oxybenzone (Swedish Research Council
2006), which suggests, in theory, that small children may not be able to eliminate oxybenzone as
easily as adults. The possibility for bioaccumulation in children, taken together with the
potential increased absorption of oxybenzone in young children (due to their greater body
surface-area-to-weight ratio) and the potential harms associated with absorption discussed above,
militates in favor of caution when using oxybenzone products in young children. Accordingly,
we are seeking any existing pediatric data on the safety of oxybenzone use in children under 2
years old. We are also requesting input on: (1) whether additional data on the safety of
oxybenzone use in young children is necessary to support the use of oxybenzone-containing
sunscreens in children under 2 years of age (taking into consideration the practical hurdles
involved in conducting studies in children of this age) or (2) whether sunscreen products
containing oxybenzone should instead be contraindicated for use in children younger than 2
years (given, among other things, the availability for use as sunscreen active ingredients of
physical UV filters like titanium dioxide and zinc oxide, which do not raise the same questions
about safe use in young children).
vii. Conclusion
Given the available data showing significant transdermal absorption and systemic
availability of oxybenzone, as well as the potential for endocrine activity, we propose that
sunscreens containing oxybenzone are not GRASE under section 505G(b)(1)(C)(ii) of the FD&C
Act because the evidence is inadequate to show that such sunscreens are GRASE. As described
above, a MUsT program should be conducted to fully characterize the absorption of oxybenzone
and to calculate a margin of safety for human use. As part of the MUsT program, we believe
that a study of oxybenzone’s metabolites in humans is also necessary; the results of this study
will inform whether additional nonclinical studies with metabolites are needed to address
potential endocrine effects. Given that oxybenzone demonstrates significant systemic
absorption, FDA believes that data on carcinogenicity (both systemic and dermal) and
Proposed Order OTC000008
Page 45
developmental/reproductive toxicity are likely to be needed to support the safety of this
ingredient, as are toxicokinetic data to bridge between animal and human data. We seek any
existing data on the pediatric safety of oxybenzone. We also seek comment on whether
additional safety data are needed to support the use of sunscreens containing oxybenzone on
children under 2 years of age, as well as comment on whether these sunscreens should be
contraindicated for use in this population. We note that, because of the risk of allergic reactions
associated with oxybenzone use, if we receive adequate data to support a positive GRASE
finding for oxybenzone, we may require labeling to address this risk. We seek comment on
whether such labeling should be required for sunscreens containing oxybenzone and, if so, what
such labeling language should entail.
In summary, table 3 shows the additional studies that FDA anticipates would be
necessary to support a positive GRASE finding for sunscreens containing oxybenzone.
Proposed Order F000001
Page 45
Table 3.--Summary of Recommendations: Studies for Oxybenzone up to 6 Percent
Safety Studies FDA Proposes Are Necessary
To Support a GRASE Determination
Additional Studies or Data Necessary?
Pharmacological Studies
Human absorption (MUsT)
(including metabolite study in humans)
Yes
Nonclinical Safety Studies
Toxicokinetics
Yes
Dermal Carcinogenicity
Yes
Systemic Carcinogenicity
Yes (FDA review of NTP studies ongoing)
DART
1
Fertility and early embryonic
development
Embryofetal development in two species
(rodent and non-rodent)
Prenatal and postnatal development
Yes
Clinical Safety Testing
Skin irritation and sensitization
No
Skin photoallergenicity and phototoxicity
No
Pediatric studies
Seeking input on whether additional studies
or contraindication are necessary to support
the safety of sunscreens containing
oxybenzone for children under 2 years of
age.
1
As noted above, if DART studies do not resolve the concerns raised in the literature relating to potential endocrine
disruption, it may be possible to resolve these concerns through additional testing.
II. Avobenzone
Our review of the available scientific literature, submissions to the sunscreen monograph
docket, and publicly available FAERS data also revealed significant gaps in the data we expect
to be necessary to support a finding that avobenzone (at up to either 3 percent or 5 percent, as
discussed below) is GRASE for use in sunscreens. While avobenzone was studied in both of the
pilot absorption studies discussed above (which concluded that avobenzone is absorbed through
the skin and into the bloodstream), we encountered no other studies examining the absorption of
avobenzone in vivo, and those in vitro studies we located had several weaknesses that limit their
usefulness in assessing the potential absorption of avobenzone from formulated sunscreen
products. In addition to the lack of pivotal MUsT studies, there are also other gaps in the record,
including (as discussed below) dermal carcinogenicity data and toxicokinetic data. Further, if
results of a sufficiently powered MUsT program demonstrate that there is significant systemic
absorption of avobenzone, or if there are adverse safety findings with avobenzone or any known
structurally similar compounds (including metabolites) in any of the other nonclinical and

Proposed Order OTC000008
Page 46
clinical studies (including an adequately conducted toxicology program), systemic
carcinogenicity and additional DART studies will be needed.
i. Background
Avobenzone, like oxybenzone, is an organic (chemical) UV filter. Because avobenzone
primarily absorbs radiation in the UVA portion of the UV spectrum, it is typically combined with
another sunscreen active ingredient that provides protection in the UVB range. Avobenzone
exhibits greater photoinstability than other UV absorbers; the available evidence shows that
avobenzone degrades quickly upon exposure to sunlight, which can cause its efficacy to be
decreased by between 50 and 90 percent after 60 minutes of exposure to sunlight (Ceresole et al.
2013; Nash and Tanner 2014).
52
To address this, avobenzone is typically combined with a
photostabilizer to prevent rapid photodegradation (Ceresole et al. 2013; Nash and Tanner 2014).
ii. Data showing transdermal absorption of
avobenzone
Although avobenzone is not soluble to any great extent in water, it is soluble in organic
solvents. These include oils (which are present on human skin), alcohols, and other substances
regularly included in sunscreen product formulations. Although this solubility is not enough, by
itself, to determine whether transdermal absorption will take place, it is a necessary precondition
(Kockler et al. 2013). In addition, like the 10 active ingredients described in section VI.C.iii.1,
avobenzone’s melting point and molecular weight are similar to those of active ingredients in
approved drug products that are successfully delivered transdermally and therefore available
systemically (Pastore et al. 2015). This potential for transdermal absorption was confirmed in
FDA’s two recent absorption studies, which showed that avobenzone is absorbed through the
skin and into the bloodstream. We were also able to locate a few studies evaluating
avobenzone’s absorption in vitro; however, these studies had a number of weaknesses that
significantly limited the conclusions that could be drawn from them.
The first in vitro study we located evaluated the penetration—through excised human
skin—of five sunscreen ingredients (including avobenzone) that had been diluted in mineral oil
and water (Hayden et al. 2005). The study used a static cell technique. As discussed in section
VI.C.iv, in a static cell study, the test product (here, a sunscreen/mineral oil/water formulation) is
placed on the upper side of a membrane (here, the excised skin) in the open donor chamber of a
static cell, and a sampling fluid is placed on the other side of the membrane in a receptor cell.
Diffusion of the ingredient (here the avobenzone) from the topically applied product to and
across the membrane is monitored by examining sequentially collected samples of the receptor
fluid. To ensure that all transdermal penetration of the ingredient that takes place is fully
52
Avobenzone’s photodegradation also results in the formation of free radicals, which could, in theory, create
sensitization and irritation responses and increase long-term risk of skin cancers and photoaging (Nash and Tanner
2014).
Proposed Order OTC000008
Page 47
reflected in the receptor fluid, the receptor fluid must be optimized for absorption (in other
words, sink conditions must be created in the fluid).
In this study, the use of skin as the membrane in the system allowed for an evaluation of
the presence and depth of permeation via skin stripping—the sequential application and removal
of adhesive tape to the skin samples. However, it is unclear whether the receptor phase of the
study created adequate sink conditions. In addition, the formulations used in the study (which, as
noted previously, consisted of only water, mineral oil, and the sunscreen ingredient) did not
contain any of the other types of excipients (such as emollients, stabilizers, or solubilizers) that
can also function as permeation/absorption enhancers and that are typically present in sunscreen
product formulations. The study results showed that there was avobenzone present in the
stratum corneum, the epidermis, and the viable dermis of the skin used as the membrane, but not
in the receptor fluid. Unfortunately, the above-described characteristics of the study limit its
value in assessing the actual absorption potential of avobenzone used in sunscreen products.
The second in vitro study (Simeoni et al. 2004) we located suffered from similar
limitations. This study assessed the avobenzone permeation observed using a static cell (as
generally described above), and then took the skin from the static cell and subjected it to multiple
rounds of tape stripping to assess the presence of avobenzone at various levels of the skin.
Following tape stripping, the skin was subjected to digestion (i.e., the skin sample was subjected
to a chemical treatment that breaks down the cell membranes to release any sunscreen that might
be either bound to proteins or bound up in the cells).
The study results showed significant retention of avobenzone in the stratum corneum, a
lesser amount in the epidermis, and none in the dermis or receptor fluid. Like the previous study,
however, the test material used in this study did not include any of the permeation enhancers
typically included in commercial sunscreen formulations. It is also unclear whether sink
conditions existed in the receptor phase of the study.
The final in vitro study used a static cell to evaluate the transdermal penetration of six
sunscreen formulations collected from a health spa that marketed its own line of skin care
products (Montenegro and Puglisi 2013). This study improved on the design of the previous two
studies in several respects. First, the receptor fluid contained ethanol, a permeation enhancer
often used in sunscreen products, which produced sink conditions in the receptor phase.
Secondly, to create favorable conditions for absorption, the products were applied at a thickness
of 20 mg/square centimeters (cm
2
) on the skin’s surface (i.e., 10 times the skin loading typically
expected (Stenberg and Larko 1985; Brown and Diffey 1986)). In addition, the study’s use of
commercially marketed sunscreen formulations (which, as discussed above, typically contain
multiple permeation/absorption-enhancing excipients) more accurately reflects the absorption
potential of marketed sunscreen products.

Proposed Order OTC000008
Page 48
Despite these improvements, the usefulness of the study was limited by its use of an
analytical method that prevented the detection of any avobenzone absorption below 100 ng/mL.
This level of absorption is hundreds of times higher than what is relevant for our considerations
in assessing the acceptable absorption level from a topically applied product. The concentration
of avobenzone used in the study (ranging from 0.2 percent to 1 percent) is also significantly
lower than what is relevant for our current consideration of maximum concentration of this
ingredient. Although avobenzone was only absorbed to a very small extent (between 3 percent
and 3.96 percent) under these study conditions, these weaknesses in the study’s design
significantly limit the conclusions that can be reached from its results.
We therefore expect that a MUsT program demonstrating the degree of absorption of
avobenzone into the human body under maximal use conditions will be needed to support a
positive GRASE determination for sunscreens containing avobenzone. We note that the in vivo
absorption studies demonstrating the transdermal absorption of avobenzone (Matta et al. 2019;
Matta et al. 2020) were pilot studies that were not sufficiently powered to eliminate the need for
a full MUsT program for this ingredient or to definitively confirm its level of absorption.
Further, in light of the above-described data showing avobenzone’s photoinstability, we also
expect that, if sufficient data are provided to support the safety of avobenzone, any future
sunscreen monograph including avobenzone as an active ingredient will include the limitation
that avobenzone is not GRASE for use in sunscreen products unless it has been photostabilized
(via use of a photostabilizing UV filter or other photostabilizing ingredient/mechanism) to
prevent its photodegradation and (among other concerns) the attendant reduction in avobenzone
efficacy.
Because photodegradation can reduce the amount of avobenzone absorbed transdermally,
we also expect that a MUsT program sufficient to support the general recognition of safety of
avobenzone for sunscreen use would need to test formulations of avobenzone that include a
photostabilizer. Including photostabilizers in MUsT formulations will allow for accurate
assessment of absorption levels in final formulated sunscreen products containing avobenzone.
This proposal is consistent with our general recommendation that materials evaluated under the
MUsT paradigm represent real-world sunscreen formulations, rather than overly simplified
solutions that fail to replicate the absorption potential of marketed formulations. As noted in
section VB.ii.2, we encourage sunscreen manufacturers to discuss their MUsT protocols with
FDA before beginning the trial.
iii. Data supporting dermal safety of
avobenzone
The available clinical dermal studies indicate that avobenzone at concentrations up to 5
percent have a favorable safety profile with respect to potential irritation, sensitization, and

Proposed Order OTC000008
Page 49
photosafety. In 2009, in conjunction with a citizen petition
53
(L’Oreal Petition, Docket No.
FDA-1978-N-0018-0675) asking FDA to take action to permit the marketing of sunscreen
products containing avobenzone up to 5 percent, L’Oreal USA Products, Inc. (L’Oreal)
submitted nine human repeat insult patch, phototoxicity, and photoallergy studies with six
different sunscreen formulations containing avobenzone (3.4 percent, 4 percent, or 5 percent).
The studies showed that the formulations were well tolerated for topical use (i.e., essentially non-
allergenic, non-irritating, and non-sensitizing, with mild to moderate reactions occurring only
rarely) (L’Oreal Petition).
54
A separate search of the available scientific literature on the clinical
safety of avobenzone did not reveal anything to undermine these findings. Although the
available literature included a small number of reports of contact irritation and photosensitization
in connection with avobenzone-containing products, details about the composition of the
formulations at issue (and the concentrations of avobenzone) were frequently missing from the
literature, making it difficult to determine the cause of these responses. A small number of
serious hypersensitivity reports for sunscreens containing avobenzone were also located in
FAERS. Because the sunscreens at issue usually contained three or more active ingredients,
however, it is difficult to determine what caused the reaction. Because sufficient data exist to
make a determination, we do not consider additional dermal clinical studies (including
photosafety, irritation, or sensitization studies) to be necessary to support the safety of this
ingredient for sunscreen use at up to 5 percent. As is customary in clinical trials, however, we
recommend that dermal safety data for avobenzone be collected during MUsT studies.
iv. Other nonclinical safety studies for
avobenzone
Dermal carcinogenicity studies have not been conducted for avobenzone. The available
data on the permeation of avobenzone suggest that it permeates into at least the dermis and
epidermis, which means that it is possible for avobenzone to impact skin tumor development.
We therefore expect that dermal carcinogenicity studies will be necessary to support a positive
GRASE finding for sunscreens containing this ingredient. Available embryofetal development
studies in rats and rabbits did not reveal any findings of concern. However, our review of the
nonclinical data for avobenzone
55
also revealed that toxicokinetic data following repeat-dose
exposure will be needed to interpret pivotal nonclinical safety studies (including the embryofetal
development studies in rats and rabbits) once the MUsT data become available. (As explained in
53
FDA-1978-N-0018-0675, two volume submission (February 20, 2009) (L’Oreal Petition).
54
Id., volume I, pp. 5-8.
55
The available nonclinical data for avobenzone include acute oral and dermal toxicity studies in rats; a 13-week
oral toxicity study in rats; a 28-day dermal toxicity study in rats; a 21-day dermal toxicity study in rabbits; several in
vitro genotoxicity tests; an in vivo micronucleus test in mice, as well as a sensitization test in guinea pigs; a primary
skin irritation test in rabbits; an ocular irritation test in rabbits; a phototoxicity study in guinea pigs; a
photoallergenicity study in guinea pigs; and embryofetal development studies in rats and rabbits (Givaudan-Roure
Petition, Docket No. FDA-1978-N-0018-0751). Importantly, (except for the embryofetal development studies)
these studies are not sufficient to resolve safety concerns for a chronically used product.
Proposed Order OTC000008
Page 50
section VI.B.ii.2, these data are used to compare drug levels achieved in animal studies with
those observed in humans under maximal exposure conditions.) In addition, if results of a
sufficiently powered MUsT program demonstrate that there is significant systemic absorption of
avobenzone, or if there are adverse safety findings with avobenzone or any known structurally
similar compounds (including metabolites) in any of the other nonclinical and clinical studies
(including an adequately conducted toxicology program), additional fertility and early embryonic
development, and prenatal and postnatal development studies in rats will be needed to support a
positive GRASE finding. Depending on the results of a sufficiently powered MUsT program
and the other factors described above, systemic carcinogenicity studies may also be needed.
v. Avobenzone in combination with other
sunscreen active ingredients
As noted in section III.B, our finding in the 1999 Final Monograph that avobenzone was
GRASE for use in sunscreens would have allowed its combination only with certain other
sunscreen active ingredients (64 FR 27666 at 27688) because we did not have targeted evidence
to support the safety and effectiveness of avobenzone when combined with the remaining active
ingredients.
We believe this limitation was inconsistent with the approach to evaluating
sunscreen combinations that the Agency has generally taken throughout the OTC Drug Review
for sunscreens. For this reason, unless evidence is submitted to suggest that there is a safety or
efficacy concern associated with the combination of avobenzone with another active ingredient,
we expect to conclude that a positive GRASE determination for avobenzone will support its use
in sunscreens either alone or in combination with all other sunscreen active ingredients.
vi. L’Oreal request to increase concentration
of avobenzone to 5 percent
Avobenzone is currently listed in the 1999 Final Monograph at concentrations up to 3
percent. As described earlier, in 2009 FDA received a citizen petition from L’Oreal requesting,
among other things, that we amend the sunscreen monograph to increase the allowable level of
avobenzone to 5 percent (L’Oreal Petition at 1). In the 1999 Final Monograph, the Agency
determined that avobenzone at concentrations up to 3 percent is an effective sunscreen active
ingredient. We now likewise conclude that the record contains sufficient information to satisfy
the effectiveness prong of the GRASE standard for sunscreens containing avobenzone at
concentrations up to 5 percent.
As described above, data submitted with that L’Oreal Petition were sufficient to establish
that avobenzone at a concentration of up to 5 percent has a favorable safety profile with respect
to potential irritation, sensitization, and photosafety. To support a finding that avobenzone at
concentrations up to 5 percent is GRASE for use in sunscreens, however, FDA expects that a
MUsT program evaluating the transdermal absorption of avobenzone up to 5 percent, as well as
Proposed Order OTC000008
Page 51
dermal carcinogenicity studies and toxicokinetic data for avobenzone at a concentration of at
least 5 percent, will also be needed.
In addition, if results of a sufficiently powered MUsT program demonstrate that there is
significant systemic absorption of avobenzone, or if there are adverse safety findings with
avobenzone or any known structurally similar compounds (including metabolites) in any of the
other nonclinical and clinical studies (including an adequately conducted toxicology program),
additional fertility and early embryonic development, and prenatal and postnatal development
studies in rats will be needed to support a positive GRASE finding. Depending on the results of
a sufficiently powered MUsT program and these other factors, systemic carcinogenicity studies
may also be needed. The record does not currently include any of these data. However, if FDA
were to receive sufficient data to support a positive GRASE finding for avobenzone up to 5
percent, we would expect to include avobenzone at this percentage in a final sunscreen
monograph.
vii. Conclusion
Given the data showing that avobenzone is transdermally absorbed, we expect that a
properly designed MUsT program will be necessary to support a positive GRASE finding for
avobenzone use in sunscreens. We expect that, to be GRASE for sunscreen use, avobenzone will
need to be photostabilized to address its potential for degradation, and we therefore expect that
any future sunscreen monograph including avobenzone as an active ingredient will include the
limitation that avobenzone is not GRASE for use in sunscreen products unless it has been
photostabilized to prevent its photodegradation. In addition, we believe that an adequate MUsT
evaluating the absorption potential of avobenzone will need to include a photostabilizer to ensure
that the potential transdermal absorption of avobenzone from avobenzone-containing sunscreens
is accurately assessed.
We also expect that dermal carcinogenicity and toxicokinetic data will be necessary to
support a positive GRASE finding for sunscreens containing avobenzone. In addition, if results
of a sufficiently powered MUsT program demonstrate that there is significant systemic
absorption of avobenzone, or if there are negative safety findings with avobenzone or any known
structurally similar compounds (including metabolites) in any of the other nonclinical and
clinical studies (including an adequately conducted toxicology program), additional fertility and
early embryonic development, and prenatal and postnatal development studies in rats will be
needed to support a positive GRASE finding. Depending on the results of a sufficiently powered
MUsT program (and the other factors described above), systemic carcinogenicity studies may
also be needed. Depending on the results of the nonclinical and pharmacology studies for this
ingredient and the safety margin that is calculated from these results, pediatric studies for
avobenzone may also be needed to support the use of sunscreens containing avobenzone in
pediatric populations.
Proposed Order OTC000008
Page 52
Table 4.--Summary of Recommendations: Studies for Avobenzone up to 3 (or 5) Percent
Safety Studies FDA Proposes Are
Necessary to Support a GRASE
Determination
Additional Studies or Data Necessary?
Pharmacological Studies
Human absorption (MUsT)
(including metabolite study in
humans)
Yes
Nonclinical Safety Studies
Toxicokinetics
Yes
Dermal Carcinogenicity
Yes
Systemic Carcinogenicity
Dependent on results of the MUsT/other safety
signals
DART
Fertility and early embryonic
development
Dependent on results of the MUsT/other safety
signals
Embryofetal development in two species
(rodent and non-rodent)
No
Prenatal and postnatal development
Dependent on results of the MUsT/other safety
signals
Clinical Safety Testing
Skin irritation and sensitization
No
Skin photoallergenicity and
phototoxicity
No
Pediatric studies
Pediatric studies may be required depending on
the outcome of the MUsT
D. Anticipated Final Formulation In Vitro Permeation Testing
As noted earlier, a final sunscreen monograph will set out the conditions under which any
product marketed pursuant to it would be GRASE. Variations among individual sunscreen
product formulations—in particular, characteristics of the specific vehicle (e.g., the cream,
lotion, or oil) in which active ingredients are delivered—can affect the transdermal absorption of
sunscreens, and thus, have an impact on their safety and effectiveness. To address this, current
and proposed monograph provisions include requirements for final formulation testing of OTC
sunscreen products to support labeled claims regarding their effectiveness—namely, testing for
SPF value as well as broad spectrum protection and water resistance where those attributes are
claimed in product labels. For purposes of this proposed order, we use the term final formulation
testing to refer to testing conducted on the sunscreen product formulation to be marketed. Our
expectation is that final formulation testing would also generally be necessary to ensure that the
active ingredient in any given sunscreen formulation permitted under the monograph would not
be systemically absorbed beyond the amount FDA determined to be safe.
Proposed Order OTC000008
Page 53
The discussion that follows provides FDA’s thinking about such testing of final
formulations, which we anticipate requiring in the future for sunscreen products marketed under
the sunscreen monograph (unless FDA determines that the ingredient or ingredients contained in
the product are unlikely to be absorbed through the skin). Because this testing would not be
required for sunscreens containing only those active ingredients proposed here as GRASE for
use in sunscreens (zinc oxide and titanium dioxide), FDA has not yet reached a final
determination as to the particular parameters that might be required for such final formulation
testing. We anticipate that we may specify final formulation testing requirements in the
monograph in the future, however, because active ingredients that we are now proposing are not
GRASE under section 505G(b)(1)(C)(ii) of the FD&C Act for use in sunscreens may be included
in the monograph in the future if FDA receives data supporting their GRASE status. Final
formulation testing requirements applicable to such ingredients would be established on an
ingredient-specific basis, taking into consideration the data provided to support a positive
GRASE determination for the specific ingredient (for example, whether any safety signals are
detected in well-conducted nonclinical carcinogenicity and DART studies). We encourage
interested parties to provide information and comment for each sunscreen active ingredient that
is relevant to establishing this kind of final formulation testing for each active ingredient.
FDA’s expectation is that this testing would not generally call for an in vivo study.
Instead, FDA expects that the conditions of marketing specified for sunscreens containing a
given active ingredient would require manufacturers to perform in vitro permeation testing
before marketing each sunscreen formulation containing that ingredient. Consistent with the
approach for SPF and broad spectrum final formulation testing set forth in the Deemed Final
Order (for proposed changes, see §§ M020.80 and M020.90 of this proposed order), FDA
anticipates that it would not review the results of the in vitro permeation testing before product
marketing. Rather, FDA expects that any future conditions pertaining to final formulation in
vitro permeation testing in the sunscreen monograph would include a requirement that
manufacturers maintain records of this testing, and that those records be available for FDA
inspection upon request.
FDA anticipates establishing a standard control formulation for each sunscreen active
ingredient to be used in the in vitro permeation testing of products containing that ingredient.
The standard control formulation would be the formulation that produces the highest in vivo
absorption in the MUsT program. The results of in vitro permeation testing using this control
formulation would then be used as a bridge to a corresponding level of in vivo absorption from
the MUsT program that is used to establish the safety margin for the ingredient. Then, FDA
anticipates establishing conditions to ensure that final formulation in vitro permeation testing
would be conducted for each formulation intended to be marketed, using the specified vertical
diffusion cell described below. The results of the in vitro permeation testing of each final
Proposed Order OTC000008
Page 54
formulation would be compared to the absorption found in the standard control formulation for
the active ingredient it contains.
In vitro permeation testing is a methodology that has been used in dermal formulation
development for over 30 years and, as used here, specifically refers to use of the “Vertical
Diffusion Cell” (Raney et al. 2008). A vertical diffusion cell is comprised of three major units:
(1) an upper chamber (into which the sunscreen formulation is placed); (2) the rate-limiting
membrane (the prepared human skin); (3) and the lower chamber/fluid channel (containing a
receptor fluid that is evaluated to determine how much of the sunscreen it “receives”) (Ng et al.
2010; Franz et al. 2009; Nallagundla et al. 2014). The vertical diffusion cell system has been
commercialized and is available as both single and multiple unit models that can be automated.
Other relevant parameters FDA expects to consider in establishing future requirements
for in vitro permeation testing include (among other things) the thickness and integrity of
collected skin, storage conditions used for collected skin, receptor fluid composition, skin and
receptor fluid temperature, the number of skin samples (and donors) used, study duration,
sampling period, application method, and number of experimenters.
We note that if a final sunscreen formulation contains a combination of sunscreen active
ingredients, FDA anticipates requiring that this final formulation be tested against the standard
control formulations for each of the sunscreen active ingredients it contains. As noted above, a
standard control formulation might not be specified for (and final formulation in vitro
permeation testing might not be necessary to establish safety for) a sunscreen containing a
particular active ingredient if FDA determines that the ingredient is unlikely to be absorbed
through the skin. As mentioned above, we therefore do not propose to require final formulation
Priorin vitro permeation testing for sunscreen formulations containing only zinc oxide and/or
titanium dioxide.
In cases in which such testing is required, FDA anticipates that if the in vitro permeation
of each sunscreen active ingredient in the final formulated product is equal to or less than the
value obtained from in vitro permeation testing of the standard control formulation for that active
ingredient, the product’s safety margin would be considered to fall within the parameters judged
to be GRASE, and thus to support marketing of the formulation. However, if the in vitro
permeation of the active ingredient from the specific final formulation is greater than the value
obtained from in vitro permeation testing of the standard control formulation for that active
ingredient, FDA anticipates that the drug product(s) using that formulation would not be
considered GRASE. In that situation, the sponsor would have the option to either: (1)
reformulate the product and conduct in vitro permeation testing to establish that the reformulated
product satisfies the final formulation in vitro permeation testing requirements set out in the
sunscreen monograph, (2) seek NDA approval for the new formulation, or (3) submit an OTC

Proposed Order OTC000008
Page 55
monograph order request (OMOR) to seek to modify the GRASE conditions in the monograph,
such as by establishing a new standard control formulation.
E. Additional Proposed Conditions of Use
i. Proposed Requirements Related to Dosage Forms
OTC sunscreens have been marketed in a variety of dosage forms over the years.
Responding in part to the growing market acceptance of spray sunscreens, on June 17, 2011,
FDA issued an ANPR addressing sunscreen dosage forms (2011 Dosage Forms ANPR) (76 FR
35669, June 17, 2011). The ANPR identified dosage forms considered eligible or ineligible for
review and potential inclusion in the OTC sunscreen monograph rulemaking under the legal
criteria then in place, based on FDA’s knowledge, at that time, of their history of marketing
before the OTC Drug Review began in 1972. It also solicited specific information about the
safety, effectiveness, and directions for use of spray sunscreens.
1. Summary of Dosage Form-Related GRASE Proposals
Prior to enactment of section 505G of the FD&C Act, a drug product was only considered
eligible for inclusion in the OTC sunscreen monograph if it was marketed OTC before the OTC
Drug Review began in 1972 or if it was determined to be eligible through submission of a time
and extent application under 21 C.F.R. 330.14.
56
In the 2019 Proposed Rule, FDA determined
that the following dosage forms were, under the paradigm in place at that time, eligible for
review and potential inclusion in the OTC sunscreen monograph based on their history of
sunscreen marketing before 1972: oil, lotion, cream, gel, butter, paste, ointment, stick, spray,
and powder. With the exception of powder, FDA proposed then and again proposes now that
sunscreens in these dosage forms are GRASE, subject to certain conditions described below and
elsewhere in this proposed order. However, as described in section VI.E.i.5, we tentatively
conclude that additional safety and efficacy data will be necessary to classify sunscreens in the
powder dosage form as GRASE and include them in the final monograph.
2. Dosage Forms That May Only Be Legally Marketed Under an
Approved Application or a New 505G Order
FDA’s determinations regarding eligibility of sunscreens in certain dosage forms in the
2019 Proposed Rule have had certain legal effects by virtue of enactment of section 505G(m)(2)
of the FD&C Act in March 2020. Specifically, sunscreens in any dosage form other than those
that FDA identified as eligible in the 2019 Proposed Rule cannot currently be legally marketed
56
We note that 21 CFR 330.14 established procedures governing the OTC drug review that have been affected by
enactment of section 505G of the FD&C Act, and that appropriate regulatory changes are forthcoming (see section
505G(k)(3) of the FD&C Act).

Proposed Order OTC000008
Page 56
without an approved NDA or abbreviated new drug application (ANDA). Sunscreens in dosage
forms that cannot currently be legally marketed without an approved NDA or ANDA (or a new
order under 505G) include sunscreen wipes, towelettes, body washes, and shampoos.
Section 505G(m)(2) provides that, “[n]otwithstanding subsection [505G](a),”
57
a drug
that, “prior to the date of the enactment of this section, the Secretary determined in a proposed or
final rule to be ineligible for review under the OTC drug review” may not be legally marketed
without an approved NDA or ANDA except as “pursuant to an order issued under this section”
(Emphasis added). In the 2019 Proposed Rule, we determined that sunscreens in all dosage
forms other than those listed above were ineligible for review under the OTC Drug Review
under the then-operative requirements because we did not receive data showing that they were
marketed prior to 1972.
58
Therefore, under section 505G(m)(2) of the FD&C Act, a sunscreen in
any dosage form other than oil, lotion, cream, gel, butter, paste, ointment, stick, spray, or powder
may only be marketed with an approved application unless FDA determines otherwise in an
order issued under section 505G of the FD&C Act. This order is not proposing to change the
non-monograph legal status of sunscreens in other dosage forms.
3. Overview of Comments on the 2011 Dosage Forms ANPR
FDA received a total of 14 comments on the 2011 Dosage Forms ANPR. Six of the
comments provided no new data, but generally supported the advantages of spray sunscreens,
agreed with the need to address concerns about spray sunscreens’ performance and/or safety
(especially when used on children), opined that existing SPF methods would not need to be
modified for sprays, or (in most cases) agreed with FDA’s suggested directions for use. Other
comments argued for the inclusion of additional dosage forms identified as ineligible in the 2011
Dosage Forms ANPR but failed to provide supporting marketing data. One comment contained
marketing information showing that sunscreen products in powder form, which we had
previously identified as ineligible for the monograph, had been marketed in the United States
before 1972, and this information supported our 2019 Proposed Rule determination that
sunscreens in powder form were eligible for consideration for inclusion in the sunscreen
monograph. The remaining comments (all from industry) provided data and information that
directly or indirectly addressed questions raised in the 2011 Dosage Forms ANPR concerning the
safety, effectiveness, and labeling of spray sunscreens. These comments are discussed in section
VI.E.i.3 below.
4. Safety and Effectiveness of Spray Sunscreens
57
We acknowledge that section 505G(a)(1)(A)(ii), which is applicable to sunscreens, addresses dosage forms.
However, section 505G(m)(2) explicitly imposes its limits on lawful marketing “notwithstanding” this provision.
58
84 FR 6024 at 6229-6230; see also 84 FR at 6206 and 6272.
Proposed Order OTC000008
Page 57
As we recognized in the 2011 Dosage Forms ANPR, compared to traditional lotions, oils,
and the like, spray sunscreens raise potential concerns of both safety and efficacy that FDA must
consider in determining whether sunscreens in the spray dosage form are GRASE. With respect
to efficacy, FDA must consider factors such as whether spraying sunscreen rather than applying
it by hand provides effective coverage on exposed skin, how consumers use spray products, and
whether current test methods for SPF and broad spectrum protection can be relied on for
adequate labeling of spray products. With respect to safety, spray sunscreens raise the question
of potential harm from inhalation of sunscreen components as well as potential flammability
risks.
I. Characteristics of sunscreen spray products
Spray sunscreens use varying technologies to package and deliver a sunscreen
formulation as an aerosol spray, i.e., an airborne suspension of fine droplets or particles. In some
spray products, the sunscreen formulation is mixed in a canister with a liquefied gas propellant
that supplies the force to generate an aerosol containing both dissolved sunscreen formulation
and propellant upon activation of a valve system. There are also pump spray sunscreen products
that are not packaged under pressure but generate spray by applied mechanical force without the
need for a propellant. Many currently marketed spray sunscreen products use a delivery
technology referred to as a bag-on-valve system, in which the sunscreen formulation is contained
in a bag with an attached valve inside a canister filled with propellant, so as not to mix the
sunscreen formulation and propellant ingredients. For purposes of this document, a spray
sunscreen product is one discharged from either a pressurized or nonpressurized container, with
the understanding that the degree of atomization will likely vary according to the formulation,
the container system used, and the design of the spray actuator, among other factors.
II. Spray sunscreen performance and
effectiveness
The Dosage Form ANPR asked a series of questions relating to the performance and
effectiveness of spray sunscreens, including questions about the amount of spray sunscreen
typically applied by consumers, uniformity of coverage, how frequently consumers reapply spray
sunscreens, whether consumers rub spray sunscreens into the skin when directed to do so and the
resulting effect on effectiveness, and whether—and if so, how—the SPF and/or broad spectrum
tests need to be modified to address sunscreen sprays. The 2011 Dosage Forms ANPR also
solicited studies comparing spray sunscreens to other eligible dosage forms to see whether the
dosage forms are comparable.
Four comments provided data from multiple studies examining and comparing the
performance of spray and lotion sunscreens on a variety of parameters, including amounts
applied, uniformity of coverage as measured with UV filter photography, comparative SPF
Proposed Order OTC000008
Page 58
results, and consumer ratings of ease and effectiveness of application, among others. FDA’s
evaluation of the information submitted indicated that key questions asked in the 2011 Dosage
Forms ANPR were directly or indirectly addressed by these studies. These studies indicated that
consumers like the convenience of spray sunscreens and adapt their use of these products to
achieve effective coverage. Data provided on application uniformity lacked study reports and
were difficult to compare directly, but—taken together—they suggest a high degree of
uniformity between sprays and lotions in coverage of exposed skin, as well as between different
spray application scenarios such as spraying directly on skin or spraying followed by rubbing.
Information submitted indicated that the amount of spray sunscreen dispensed is higher than the
amount dispensed for sunscreen lotions, and that consumers are more likely to reapply sprays
than lotions. There was no response from any stakeholder regarding consumers’ compliance
with directions to rub a spray sunscreen into the skin. However, data was provided suggesting
that rubbing spray sunscreens into the skin did not enhance effectiveness. Based on these
comments and the available data, we are not proposing to require that labeling provide
instructions to rub spray sunscreens into the skin.
Comments on the 2011 Dosage Forms ANPR also agreed, and we concur, that the SPF
and broad spectrum tests applicable to sunscreens in other dosage forms are appropriate for
assuring the efficacy of sunscreens in spray dosage forms. SPF testing requires application of a
set amount of sunscreen (2 mg/cm
2
on each test subject), which can readily be done for spray
sunscreen formulations. For example, comments on the Dosage Form ANPR stated that the SPF
testing of sunscreen spray products can be conducted following the method described in what is
now the Deemed Final Order by weighing out the liquid form and applying it to the skin. (See §
MO20.80(d.)) This premise is supported by data from SPF testing submitted in the comments.
For example, one comment submitted five SPF testing reports conducted on sprays using the
FDA-required methods, in which the expected SPF values for the test formulations were almost
identical to the SPF testing results. The same logic applies to broad spectrum testing, which also
uses a defined amount of sunscreen by weight. Based on this information, we conclude that the
proposed SPF and broad spectrum testing methods are also appropriate for spray dosage forms.
III. Spray sunscreen safety
FDA has identified two primary safety concerns specific to spray sunscreen dosage
forms: (1) the potential risk of respiratory harm from inhaling sunscreen ingredients and (2) the
potential flammability risk when consumers are exposed to flame or heat before spray solvents
have completely dried. For the reasons described below, we believe that both potential risks can
be acceptably mitigated by proposed formulation limitations, labeling requirements, and
adequate testing, and thus propose to establish these as additional conditions in the monograph to
ensure that sunscreen products in a spray dosage form would be GRASE.
IV. Inhalational toxicity
Proposed Order OTC000008
Page 59
Broadly speaking, the human respiratory system consists of the upper respiratory tract
(i.e., the airways of the nose to the larynx) and lower respiratory tract (the trachea and branching
airways of the lung, including bronchi, bronchioles, and alveoli) (see generally Stuart 1984;
Leikauf 2013). Much of the respiratory system is lined with a layer consisting of mucus cells
and cilia that mechanically propel inhaled particles out of the lower respiratory tract toward the
mouth, where they may be swallowed or expectorated (Stuart 1984; Leikauf 2013). The most
significant concern associated with any product that may be accidentally inhaled is the potential
risk of adverse effects associated with deep lung deposition, which occurs when particles in an
aerosol (i.e., a suspension of airborne particles such as a sunscreen spray) reach the unciliated
airways in the lung. Particles that can reach the unciliated airways of the deep lung are described
as respirable and may be associated with serious adverse effects such as asthma, emphysema,
bronchospasm, or chronic obstructive pulmonary disease; particles that do not reach the deep
lung may be associated with less harmful adverse events such as local irritation of the upper
airway, coughing, or sneezing (Leikauf 2013; Rothe et al. 2011; Steiling et al. 2014). The
potential health risk associated with inhalation of hazardous aerosols depends on how much of a
toxic substance is deposited in a given region of the respiratory tract and how much remains after
physiological clearance occurs through mechanisms such as coughing, sneezing, mechanical
transport, or, in the deep lung, engulfment by specialized cells or other protective action (Stuart
1984; Brown et al. 2013).
The pathogenic potential of inhaled aerosols depends on where in the respiratory tract a
particle is deposited (Brown et al. 2013). Whether spray particles that enter the body through
inhalation at the nose or mouth will be deposited in the lung depends largely on their physical
characteristics: most notably particle size, with the likelihood of respirability increasing as
particle size decreases (Stuart 1984; Leikauf 2013; Rothe et al. 2011; Steiling et al. 2014; Brown
et al. 2013; Liu et al. 2017). The effects of particle size on respirability of inhaled particles is
well studied. There is general agreement that particles greater than 10 micrometers (µm) in
diameter may enter the mouth and the airway up to the larynx. Approximately 50 percent of
particles up to 10 µm in diameter can penetrate beyond the larynx to the thoracic region of the
respiratory tract, while only particles smaller than 4 µm reach the unciliated airways and alveolar
region of the lungs (see generally Stuart 1984; Leikauf 2013; Rothe et al. 2011; Steiling et al.
2014; Brown et al. 2013; Liu et al. 2017). Thus, although there are little or no data on the
potential inhalation toxicity of particular spray sunscreen ingredients, we are proposing that
exposure to harmful levels of such ingredients can effectively be minimized by imposing particle
size limitations on spray sunscreen products.
Several comments on the 2011 Dosage Forms ANPR submitted results of particle size
distribution testing using available methods and apparatus, with the aim of showing that
exposure to inhaled sunscreen products or ingredients would be minimal and thus unlikely to
cause adverse effects. The data submitted were similar and in some cases overlapping. In an
Proposed Order OTC000008
Page 60
analysis of pooled particle size distribution data from all submissions, representing 50 U.S.-
marketed spray sunscreen products, 32 had particles smaller than 4 µm in diameter and thus
within the respirable portion of the total particle size distribution. However, the great majority of
the particle sizes observed were nonrespirable. The highest percentage that any product had of
particles smaller than 4 µm in diameter was 0.43 percent and the mean was 0.22 percent, which
is extremely low.
In addition to reviewing information from comments on the 2011 Dosage Forms ANPR,
FDA conducted its own analysis of particle size distribution for 14 marketed spray sunscreens.
In those tests, no sunscreen had more than 10 percent of particles in sizes less than 10 µm in
diameter and only three had particles smaller than 5 µm (Liu et al. 2018).
To limit the risks of unintentional exposure and potential associated adverse events to
respirable particles in spray sunscreens, we are proposing limits on the size of particles dispensed
from the consumer container for finished spray sunscreens in order for those products to be
GRASE. We propose that 90 percent of the particles dispensed from the consumer container
must be at least 10 µm or greater in order to limit exposure beyond the larynx, and to prevent
deposition in the deep lung, the minimum particle size dispensed from the consumer container
must be no less than 5 µm. This limit was chosen because it is the lowest whole number above
the generally accepted threshold (4 µm) at which particles enter the unciliated airway and
because it allows for experimental error that may be inherent in particle size measurements.
Sunscreen products that do not meet both limitations would not be GRASE because the evidence
is inadequate to support a positive finding about their safety (see section 505G(b)(1)(C)(ii) of the
FD&C Act). We believe that, taken together, these two limitations would significantly reduce
inhalation risk from spray sunscreens by reducing particle exposure to the larynx and deeper lung
tissues.
With the establishment of these two limits, FDA believes that the risks of adverse events
related to unintentional inhalation of spray sunscreens will be minimal. Stakeholders asserted
that the risk of inhalation toxicity is already low, primarily based on particle size of marketed
sprays. Limited data on adverse event reports and animal toxicity studies were also submitted in
a few comments on the 2011 Dosage Forms ANPR, but were inadequate to support the safety of
spray sunscreens in the absence of particle size limitations. If the particle size limitations
proposed here are adopted, however, we do not believe that additional animal toxicity or other
safety data need to be provided to support a GRASE finding for spray sunscreens.
We are proposing that particle size testing to demonstrate compliance with the proposed
limitations must be conducted on spray products as they are dispensed from the consumer
container as part of the lot release testing that would be routinely completed as part of current
good manufacturing practice (CGMP) compliance under part 211 (21 CFR part 211). It is
Proposed Order OTC000008
Page 61
necessary to test the size of particles dispensed from the consumer container to ensure that
particle size requirements are met under conditions of use by consumers.
For purposes of these proposed particle size requirements, we are using the term particle
size broadly to mean the discrete unit emitted from the spray container that is available for
inhalation by a consumer when the product is applied. If the particle dispensed from the
consumer container is a droplet that meets the size requirements, the consumer will not
accidentally inhale it into the deep lung. However, if that same droplet breaks apart into smaller
fractions when it is dispensed from the consumer container, those fractions would be the
particles that must meet the size requirement to ensure that consumers will not inadvertently
inhale them past the larynx.
We are not proposing a specific test methodology for spray sunscreen particle size.
Rather, sunscreen manufacturers would be obligated to ensure that particle size testing for their
sunscreen sprays would be conducted on each lot of the final product as dispensed from the
consumer container in accordance with adequate written specifications. U.S. Pharmacopeia
(USP) General Chapter 429 provides methodology and requirements for sprays, aerosols, and
powders that include methodology to determine droplet/particle size distribution, and we expect
to consider testing done in accordance with the USP as adequate to meet this proposed
requirement (U.S. Pharmacopeia 2021).
We note that several comments on the 2011 Dosage Forms ANPR expressed concern
about the potential inhalation risk from exposure to spray sunscreens that contain nanomaterials
(as both active and inactive ingredients). One comment also recommended that FDA require the
presence of such ingredients to be disclosed on spray sunscreen labels. FDA’s approach to
nanotechnology and nanomaterials in sunscreen products is discussed in section VI.B.v. FDA is
not now proposing conditions of use for spray sunscreens, including labeling, that distinguish
based on the presence of nanomaterials because we are proposing that any sunscreen spray that
contains any particles smaller than 5 µm when it is dispensed from the consumer container
would not be GRASE. With respect to nanomaterials in spray sunscreens, we note that the
primary determinant of inhalation risk is the size of the particles in emitted sprays, which may be
larger than individual formulation components. Nanoscale ingredients would not pass the
particle size limitations for spray sunscreens; therefore, if they were to be detected when sprayed
from the consumer container during particle size testing, the sunscreen could not be marketed
under the OTC monograph.
In addition to the proposed limitations on particle size for sunscreen sprays and related
testing, we are proposing to require that the following labeling be included in the directions for
sunscreen sprays to minimize unintended inhalation:
Proposed Order OTC000008
Page 62
• Hold container 4 to 6 inches from skin to apply.
• Do not spray directly into face. Spray on hands then apply to face.
• Do not apply in windy conditions.
• Use in a well-ventilated area and avoid inhalation.
This language is the same as that published in the 2011 Dosage Forms ANPR. Its
adoption was supported by comments on the 2011 Dosage Forms ANPR, and the language is
widely used on currently marketed spray sunscreens.
V. Flammability risk
In July 2013, FDA issued a Consumer Update regarding persons catching on fire while
wearing spray sunscreen products near an open flame:
The Food and Drug Administration (FDA) has become aware of five separate
incidents in which people wearing sunscreen spray near sources of flame suffered
significant burns that required medical treatment. The specific products reported
to have been used in these cases were voluntarily recalled from the market, so
should no longer be on store shelves.…In the five incidents reported to FDA,
however, the burns occurred after the sunscreen spray had been applied. The
ignition sources were varied and involved lighting a cigarette, standing too close
to a lit citronella candle, approaching a grill, and in one case, doing some welding
(FDA 2013).
These cases all involved a single manufacturer’s product that has since been voluntarily
recalled. However, sunscreens are often used in very hot outdoor environments with high
ambient air temperatures. Sunscreens are also frequently used around sources of flame or sparks,
such as grills, bonfires, smoking, or other ignition sources. To ensure safe use of spray
sunscreens and to better inform consumers about potential flammability risks, we are proposing
to limit the flammability and require flammability labeling of spray sunscreens under the OTC
sunscreen monograph.
FDA’s general labeling regulations for OTC drugs provide for OTC monographs to
require flammability labeling in suitable cases (21 CFR 201.66(c)(5)(ii)(C)), and we have done
so for products such as topical antitussives (M012) and wart removers (M028). As we did for
those products, we are proposing to require each spray sunscreen formulation to be labeled for
flammability in accordance with the testing methodology described in a regulatory provision
issued by the Consumer Product Safety Commission (CPSC) (see 16 CFR 1500.43a). We have
proposed to incorporate this flash point testing methodology to address our concern regarding the
flammability of sunscreen in the spray dosage form after it has been dispensed onto the skin. We
therefore propose that all batches of sunscreen spray products be tested for flammability in
Proposed Order OTC000008
Page 63
accordance with 16 CFR 1500.43a as part of batch release testing conducted in accordance with
CGMP requirements. Because regulations may change over time, the proposed order specifies
that these tests must be performed using the procedure as described when the regulation was first
published on August 8, 1986 (51 Fed. Reg. 28539).
We are also proposing to define three flammability categories for use in regulating and
labeling sunscreens: (1) extremely flammable, (2) flammable, and (3) combustible. These
definitions refer to flash point testing to be performed using the method described in 16 CFR
1500.43a. These definitions are analogous to certain CPSC definitions located at 16 CFR
1500.3. Given the conditions under which sunscreens may be used, we are proposing that spray
sunscreens found to meet the definition of extremely flammable in proposed § M020.3(d) are not
GRASE and may not be marketed under the OTC sunscreen monograph because the evidence
shows that such sunscreens are not GRASE under section 201(p)(1) of the FD&C Act (see
section 505G(b)(1)(C)(i) of the FD&C Act). Products found to meet the definition of flammable
or combustible in proposed § M020.3(e) or (f) would be required to include the following
language in the “Warnings” section of the drug facts labeling: [bullet] “Flammable” or
“Combustible” [as applicable] followed by a colon and the statement “Keep away from fire or
flame.”
A further concern related to flammability is the time required for volatile solvents in a
spray product to dry on the skin before a consumer can safely approach a source of heat or flame
or can smoke without danger of fire. Typical sunscreen spray formulations contain 50 to 80
percent of a volatile carrier, most commonly ethyl alcohol. These volatile solvents are necessary
to the formulation to allow the product to be sprayed onto the skin. After spraying, the solvents
are intended to rapidly evaporate leaving a film of UV filters on the skin surface as the product
dries. Once a spray product is dry, the solvent is no longer present, so the flammability risk is
low. However, prior to this point, the flammability risk would be higher.
We think that consumers should be warned to stay away from sources of flame while a
flammable or combustible sunscreen spray dries. For this reason, we propose to require that
each batch of a sunscreen spray product that meets the definition of flammable or combustible in
§ M020.3(e) or (f) be tested for drying time in accordance with written specifications. If the
drying time is less than 5 minutes, we propose to require that the labeling state, “Wait 5 minutes
after application before approaching a source of heat or flame, or before smoking.” If the drying
time is at least 5 minutes but less than 10 minutes, we propose that the labeling would state,
“Wait 10 minutes after application before approaching a source of heat or flame, or before
smoking.” We propose that a sunscreen spray that is flammable or combustible and that takes 10
minutes or more to dry would be not GRASE under section 505G(b)(1)(C)(i) of the FD&C Act
because of the possibility of consumers approaching sources of fire during such an extended
drying period. We invite comment on this approach.
Proposed Order OTC000008
Page 64
5. Powder Dosage Form
We have tentatively determined that additional data as outlined below will be needed to
support a conclusion that sunscreens in powder dosage form are GRASE and to support
consideration of appropriate labeling. For this reason, we propose that powder sunscreens are
not GRASE because the evidence is inadequate to show that they are GRASE under the FD&C
Act section 201(p)(1) (see section 505G(b)(1)(C)(ii) of the FD&C Act). Also, like sprays,
powder sunscreens pose the potential for unintended inhalation, and for this reason, if admitted
to the sunscreen monograph, the same limitations as to particle size here proposed for sprays
would be expected to apply. For powder sunscreens that meet the particle size limitations
proposed for sprays, we do not expect that additional toxicology data would be needed to address
the potential health risks associated with inhalation.
One comment on the 2011 Dosage Forms ANPR provided data on SPF and broad
spectrum performance of five powder formulations, as well as data from repeated insult patch
tests and photosensitivity studies that were asserted not to show any safety issues. FDA has
conducted particle distribution testing on five powder sunscreens. The powder sunscreens tested
had a larger proportion of relatively small particles compared to the sprays. Only one of the five
powder sunscreens would have complied with the requirement we are considering that no more
than 10 percent of the particles could be smaller than 10 µm in diameter, and that product was
also the only one that would have met the prospective limitation of no particles smaller than 5
µm in diameter (Liu et al. 2017; Liu et al. 2018). Based on the data submitted, we believe that
the SPF and broad spectrum test methods used for other sunscreen dosage forms are appropriate
for use with powder sunscreens, and we are not requesting additional respiratory safety
information for powders that meet the same particle size limitations proposed for spray
sunscreens.
FDA invites comments and data on the following topics related to powder sunscreens:
• What amounts of powder sunscreens do consumers typically dispense?
• What amounts of powder sunscreens are effectively transferred to the skin?
• How uniform is the sunscreen application across the sun-exposed area of the
skin?
• How frequently do consumers reapply the product?
• Does rubbing a powder into the skin change sunscreen effectiveness?

Proposed Order OTC000008
Page 65
• Are powder dosage forms water-resistant? If they are not water-resistant, is a
direction to reapply every 2 hours sufficient to assure their safe and effective
use?
• Can the powder dosage form be used safely and effectively over all areas of
skin exposed to the sun, or should this dosage form be limited to the face?
• What factors, if any, should FDA consider in connection with particle size
limitations or test methods for sunscreen powders?
• Are there important differences among powder types (e.g., loose, compact) or
applicators that would affect particle size testing?
FDA will evaluate data and information submitted in response to these questions, as well
as any other submitted data, to determine whether they support a final determination that
sunscreens in this dosage form are GRASE.
ii. Proposed Maximum SPF and Broad Spectrum Requirements
In the 1999 Final Monograph, FDA established SPF 30+ as the maximum labeled SPF
value for sunscreen monograph products and required that each sunscreen monograph active
ingredient contribute a minimum SPF of 2 to finished sunscreen products (64 FR 27666 at
27672, 27674, and 27675). The final monograph did not include any broad spectrum protection
provisions. In its 2001 decision to stay the final monograph, however, FDA indicated that it was
issuing the stay because the Agency intended to amend the sunscreen monograph to address
requirements for both UVA and UVB radiation protection (66 FR 67485). FDA later addressed
these issues in the 2011 L&E Final Rule, which, among other things: (1) established optional
broad spectrum labeling based on satisfaction of a critical wavelength test, (2) created an
optional indication relating to skin cancer and early skin aging risk reduction for broad spectrum
products with an SPF of 15 or higher, and (3) required a labeling warning for sunscreens that did
not both satisfy the broad spectrum test and provide an SPF of at least 15 (76 FR 35620 at
35626-35628). Concurrently with publication of the L&E Final Rule, FDA issued a proposed
rule to raise the maximum labeled SPF value for sunscreen products containing sunscreen
monograph active ingredients to SPF 50+ (76 FR 35672, June 17, 2011).
In March of 2020, the CARES Act was passed. The Deemed Final Order for sunscreens
established by the CARES Act does not include a limit on maximum SPF values.
59
In the time
59
Specifically, Congress established that as a baseline, “the applicable requirements in terms of conformity to a final
monograph” for sunscreens are “the requirements specified in [the 1999 published version of 21 CFR part 352],
except that the applicable requirements governing effectiveness and labeling shall be those specified

Proposed Order OTC000008
Page 66
since FDA’s 2011 publications, the body of evidence in the published literature on UVA
radiation (particularly UVA I radiation) and its role in the development of skin cancer has grown.
This new data about the harms of UVA exposure is a significant concern given, among other
things, that with currently available sunscreens, consumers may unknowingly accumulate
excessively large UVA doses by using sunscreens with high SPF values that either: (1) do not
pass FDA’s current critical wavelength-based broad spectrum test or (2) have inadequate
uniformity in their UVA protection. Because of these concerns, we are making a number of
proposals designed, among other things, to couple a greater magnitude of UVA protection to
increases in SPF values.
1. Background
UV radiation includes both UVA and UVB rays. UVB rays (i.e., those with wavelengths
from 290 to 320 nm) are higher energy, are much more effective at producing sunburn, and
produce greater amounts of cellular damage (including DNA lesions, which can result in gene
mutations linked to skin cancers) (Mouret et al. 2006; McKinlay and Diffey 1987). UVA rays
(i.e., those with wavelengths from 320 to 400 nm) are lower energy and less effective at
producing sunburn, but make up the majority of UV radiation, and penetrate much deeper into
the skin, potentially causing oxidative damage (through formation of ROS) to skin pigment cells
(Noonan et al. 2012). UVA rays also contribute to photo-aging (Mouret et al. 2006; Ridley et al.
2009). Although the current scientific literature attributes UV-signature DNA lesions primarily
to UVB wavelengths, UVA wavelengths can also produce DNA lesions. Although UVA
wavelengths produce DNA lesions to a significantly lesser degree than UVB wavelengths do,
DNA lesions produced by UVA rays have been reported to have slower repair rates (Mouret et
al. 2006). UVA rays are comprised of UVA I rays (340 to 400 nm) and UVA II rays (320 to 340
nm). As discussed below, until recently, UVA I rays were generally not considered to contribute
significantly to the harms associated with UV exposure.
Sunscreen products must be labeled with an SPF value calculated using a standardized
SPF testing procedure set forth in the Deemed Final Order at § M020.80. The SPF test measures
the amount of UV radiation exposure it takes to cause sunburn when a person is using a
sunscreen compared with how much UV exposure it takes to cause sunburn when the person is
not using a sunscreen. Sunscreens with increasing SPF values (up to a certain point) have been
demonstrated to provide increased sunburn protection. Because SPF values represent a
sunscreen’s level of sunburn protection, they are primarily (though not exclusively) an indicator
of expected protection from UVB radiation. To pass FDA’s current test for broad spectrum
in [21 CFR] section 201.327” (section 505G(a)(2) of the FD&C Act). The only provisions in the 1999 version of
part 352 that addressed maximum SPF were labeling provisions (see 64 Fed. Reg. 27662 at 27688, establishing 21
CFR 352.50); they have therefore been superseded by the labeling provisions in 21 CFR 201.327, which did not
include any maximum labeled SPF provisions.
Proposed Order OTC000008
Page 67
labeling (Deemed Final Order at § M020.90), however, sunscreens must demonstrate that, in
addition to UVB protection, they also provide some UVA protection.
Only products that have been determined to have a minimum SPF value of 15 and to pass
our broad spectrum test may include statements in their labeling indicating that they decrease the
risk of skin cancer and early skin aging caused by the sun when used as directed with other sun
protection measures (Deemed Final Order at § M020.50(c)(2)). In contrast, sunscreens that have
not been determined to provide both broad spectrum protection and an SPF value of at least 15
must include a skin cancer/skin aging alert warning to consumers that “[s]pending time in the
sun increases your risk of skin cancer and early skin aging” and that “[t]his product has been
shown only to help prevent sunburn, not skin cancer or early skin aging” (Deemed Final Order at
§ M020.50(d)(2)).
2. Increased Evidence of Harms Associated with Exposure to UVA
Radiation
Since publication of the 2011 L&E Final Rule and 2011 Max SPF PR, the strength of
scientific evidence linking UVA exposure to skin cancers and other harms has increased. This
evidence suggests that UVA wavelengths continue generating DNA lesions hours after UV
exposure (Premi et al. 2015) and that if left unrepaired, these DNA lesions can form UV-induced
mutations in many genes that have been detected in both melanoma and nonmelanoma skin
cancers (Brash 2016; Hodis et al. 2012; Krauthammer et al. 2012; Ziegler et al. 1994). Further,
unlike UVB-induced DNA lesions, which attenuate with skin depth, recent evidence indicates
that DNA lesions induced by UVA I exposure show the opposite pattern, with both increased
DNA lesions in the basal layer of the epidermis (where melanocytes and proliferating
keratinocytes reside) and less efficient DNA lesion repair in the basal layer (Tewari et al. 2013;
Tewari et al. 2012).
Damage to cells in the basal layer (if left unrepaired or if inefficiently repaired) can lead
to mutations in critical genes that increase the possibility that normal cells will transform into
cancer cells. While inefficient DNA repair is a concern for all individuals exposed to UV
radiation, this concern is particularly acute in those with xeroderma pigmentosum (a disease
caused by a disorder of the DNA repair system), who have extreme sensitivity to UV radiation,
and who develop both nonmelanoma skin cancer and melanoma with a high frequency and very
early in life (DiGiovanna and Kraemer 2012). In addition to the skin cancer-related risks
associated with UVA exposure, increasing evidence shows that UVA I radiation also produces
immunosuppression (Damian et al. 2011; Marionnet et al. 2014). This, too, is a general concern
for all individuals, but is especially dangerous for certain at-risk populations (such as organ
transplant recipients and others on immunosuppressive drugs).

Proposed Order OTC000008
Page 68
Given the above-described evidence, we are concerned about the existing potential for
inadequate UVA protection in marketed sunscreen products. This is a particular concern with
respect to high SPF sunscreen products that do not pass FDA’s current critical wavelength-based
broad spectrum test or that (though they pass our current broad spectrum test) have inadequate
uniformity in their UVA protection. Consumers using these products may, while successfully
preventing sunburn, accumulate excessively large doses of UVA radiation, thereby exposing
themselves to additional risks related to skin cancer and early skin aging. The International
Agency for Research on Cancer has found that high SPF sunscreen products are associated with
longer intentional UV exposures (Autier et al. 2007), raising the concern that use of these
products may result in significant doses of UVA radiation. We note that concerns relating to
inadequate UVA protection came up in several comments we received in response to the 2011
Max SPF PR, and that these comments raised particular concerns about inadequate UVA
protection in high SPF products. This concern has also grown over time in the published
literature (Diffey 2012; Diffey 2009; Wang et al. 2008; Diffey 2001).
For all of these reasons, we are proposing a number of steps designed to couple a greater
magnitude of UVA protection to increases in SPF values. As discussed in further detail below,
we are also making proposals designed to address evidence of variability in SPF values and
evidence showing additional clinical benefits associated with SPF 60 sunscreens.
3. Broad Spectrum Proposals
I. UVA I/UV ratio required to pass the broad
spectrum test
We are proposing certain changes to the requirements to pass the broad spectrum test.
Specifically, we are proposing to add to the current broad spectrum test a requirement that
products meet a UVA I/UV ratio of 0.7 or higher. We note that the current broad spectrum test
procedure would remain unchanged
60
and that this new ratio would be calculated using data from
the existing test.
FDA currently requires that sunscreens labeled as broad spectrum achieve a critical
wavelength of 370 nm or greater (Deemed Final Order at § M020.90). A sunscreen product’s
UV protection is often displayed as a curve on a graph showing the amount of UV absorbance
the product provides at each wavelength in the UV spectrum (i.e., from 290 to 400 nm). The
“critical wavelength” of the product is the wavelength corresponding to 90 percent of the area
60
We note that, as described in section VI.E.iv.2.IX, we are proposing a minor revision in equipment specifications
for the broad spectrum test to respond to feedback that FDA received on this issue and proposing some minor
revisions to current language to make clear our existing expectations.
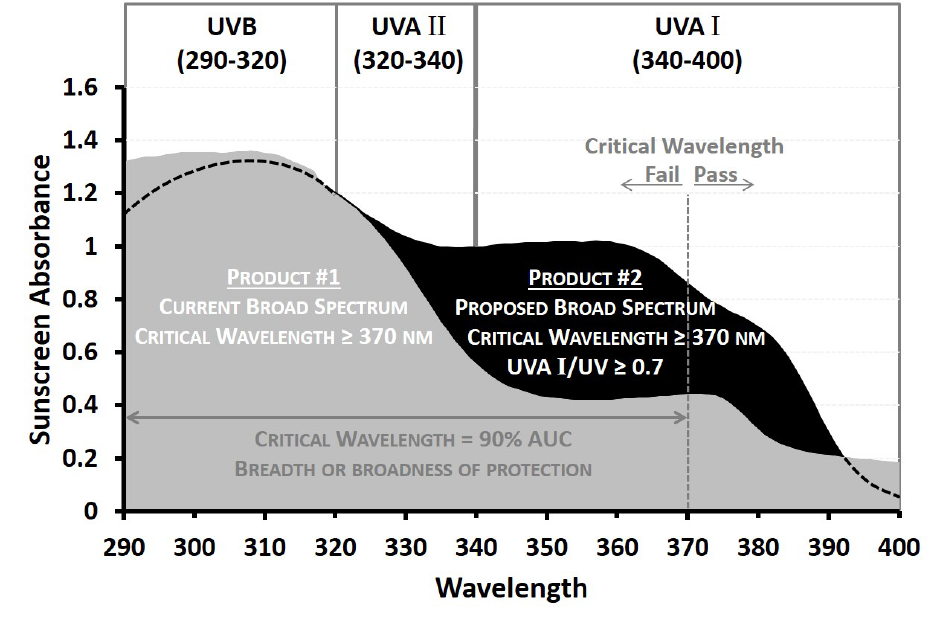
Proposed Order OTC000008
Page 69
under this curve. Higher critical wavelengths, therefore, illustrate greater breadths of UV
protection across the 290 to 400 nm spectrum.
Most sunscreen products—even if they achieve a critical wavelength of 370 nm or
greater and therefore meet the current criteria for broad spectrum labeling—have historically
covered the UVB and UVA II ranges preferentially. Given how much of the UVA portion of the
UV spectrum is composed of UVA I radiation (see Figure 3 below) and given what we now
know about the risks associated with UVA exposure, and with UVA I exposure in particular,
ensuring that sunscreen products provide adequate protection in the UVA I portion of the
spectrum is critical.
Figure 3.--Comparison of Two SPF 15 Products
1
1
Product 1 satisfies the current broad spectrum requirement of achieving a critical wavelength of 370 nm or greater;
product 2 meets both components of the proposed broad spectrum requirement (achieving both a critical wavelength
of 370 nm or greater and a UVA I/UV ratio of 0.7 or greater).
We are therefore proposing to require that in order to pass the broad spectrum test, a
product must demonstrate that it provides a UVA I/UV ratio of 0.7 or higher, indicating that the
product provides a minimum measure of UVA I radiation absorbance relative to total UV
radiation (i.e., UVB + UVA) absorbance, in addition satisfying to the 370 nm critical wavelength
requirement. Requiring a UVA I/UV ratio of 0.7 or higher for broad spectrum products would
Proposed Order OTC000008
Page 70
mean that these products would have a more uniform amount of radiation protection across the
UVA I, UVA II, and UVB ranges. This improved fidelity across the UV spectrum is especially
important for high SPF products which, as discussed above, are associated with longer
intentional sun exposure, which in turn can result in significant doses of UVA radiation. This
proposed UVA I/UV ratio would also help eliminate the current potential for a product labeled as
broad spectrum that has a higher SPF value to provide (unbeknownst to the consumer) poorer
broad spectrum protection than a product labeled as broad spectrum with a lower SPF value
(depending on the particular combination of active ingredients used in the product and which
parts of the UV spectrum they absorb). For example, under the current testing regime, a
sunscreen that is labeled “broad spectrum SPF 30” could provide less UVA protection than a
sunscreen labeled “broad spectrum SPF 15.”
We note that FDA first raised concerns relating to the adequacy of UVA protection in
sunscreen products in 2007 (see 72 FR 49070 at 49104 to 49107). At that time, we proposed a
similar ratio to the one we are proposing today as part of a different, more complex proposal for
testing and labeling to address broad spectrum protection that, among other things, included both
in vitro (spectrophotometric) and in vivo (clinical) testing for UVA radiation, as well as a four-
tier UVA star rating labeling system. In response to comments describing purported
disadvantages of that proposal, including general comments that the proposal was
overcomplicated, specific comments on the proposed in vitro testing method, and comments
indicating that “[t]he proposed ratio places too much emphasis on the UVA I region, which is not
generally considered to contribute significantly to the harmful effects of exposure to UV
radiation” (76 FR 35620 at 35650), we made a number of changes to our 2007 proposal in the
2011 L&E Final Rule. Those changes included elimination of the UVA I/UV ratio and adoption
of the above-described critical wavelength test for establishing broad spectrum protection
instead. As we noted in the preamble to the L&E Final Rule, our decision not to require the
UVA I/UV ratio at that time was based, in part, on our agreement with comments stating that the
scientific evidence available at that time indicated that UVA I exposure did not pose sufficient
risk of harm to justify the emphasis placed on it by the ratio, and that the critical wavelength test
provided a superior measure of broad spectrum protection (id. at 35650).
As described above, in the time since issuance of the L&E Final Rule, the body of
evidence showing the harms of UVA exposure, and of UVA I exposure, in particular, has grown
significantly (Noonam et al. 2012; Premi et al. 2015; Brash 2016; Tewari et al. 2013; Tewari et
al. 2012; Damian et al. 2011).
It is now clear that in addition to producing the
immunosuppression described above, UVA I exposure also results in increasing DNA damage
with increasing skin depth (in contrast to UVB-induced DNA damage, which is reduced as skin
depth increases). In addition, given that UVA I is the predominant portion of UVA radiation,
new evidence (discussed in section VI.E.ii.2) strengthening the link between UVA radiation and
skin cancer development raises our concerns about the potential for inadequate protection in the

Proposed Order OTC000008
Page 71
UVA I portion of the UV spectrum. Accordingly, we no longer agree with the earlier comments
suggesting that UVA I does not contribute significantly to the harmful effects of exposure to UV
radiation, or with our 2011 conclusion that a UVA I/UV ratio requirement would therefore place
too much emphasis on this portion of the UV spectrum.
We emphasize that we are not proposing to replace the existing critical wavelength test,
and that the proposed ratio would supplement (and be calculated using data from) the existing
broad spectrum test. We also note that the UVA I/UV ratio we are proposing would result in a
level of UVA protection similar to what is achieved via the European Union’s recommended
minimum UVA protection factor of 1/3 of the labeled SPF and via the United Kingdom’s Boots
3-star rating (the United Kingdom has for decades used a tiered star rating system based on an
alternative ratio method) (Diffey 2009; Wang et al. 2008). We note that data collected in 2009
about 330 sunscreen products commercially available in the United States showed that, at that
time, more than half of these products already satisfied the broad spectrum test we are now
proposing (see Comment, Docket No. FDA-1978-N-0018-0690).
II. Broad spectrum requirement for all products
that are ≥ SPF 15
We are also proposing to require that all sunscreen products with SPF values of 15 and
above demonstrate that they provide more uniform protection across the UVA I, UVA II, and
UVB ranges of the UV spectrum by satisfying FDA’s revised broad spectrum test. This proposal
is designed to link increases in SPF value not only to increases in UVB protection, but to
increases in the magnitude of UVA protection as well. We note that a consumer using a
sunscreen that provides robust protection against sunburn but that does not pass FDA’s revised
broad spectrum test—and therefore provides inadequate UVA protection—may fail to get out of
the sun, thereby exposing themselves to higher levels of UVA radiation than if they had not been
protected from sunburn. Given the increasing evidence of major health risks associated with
UVA exposure, we propose to find that such products (those with SPF values of 15 and greater
that do not provide sufficient protection across the UV spectrum (as demonstrated by satisfying
FDA’s revised broad spectrum requirement)) are not GRASE. See section 505G(b)(1)(C)(i) of
the FD&C Act. At the same time, we conclude that the evidence described above regarding the
contribution of UVA I to skin carcinogenesis, coupled with the evidence reviewed in the 2011
L&E Final Rule (see 76 FR 35620 at 35630-35634), supports the proposal to include sunscreen
products that have an SPF of 15 or higher and also pass the revised broad spectrum test in the
sunscreen monograph with indications both for use to help prevent sunburn and for use, as
directed with other sun protection measures, to reduce the risk of skin cancer and early skin
aging caused by the sun.
61
61
We propose that such products would be GRASE under section 201(p)(1) of the FD&C Act. See section
505G(b)(1)(A) of the FD&C Act.

Proposed Order OTC000008
Page 72
As we indicated in the L&E Final Rule, the whole range of UV radiation, and not specific
wavelengths, is a human carcinogen, and the exact wavelengths most responsible for these
harmful effects are not known (see id. at 35631, 35633). To assure that a clinically meaningful
reduction in the risks of skin cancer and early skin aging is achieved, then, a product must
contribute to substantially limiting overall UVB and UVA exposure (see id. at 35630, 35631-
35632), as will be assured by our proposal to couple the enhanced breadth of protection across
the UVA spectrum provided by the revised pass criteria for the broad spectrum test with the
magnitude of protection assured by requiring a minimum SPF of 15.
By requiring that all sunscreens with SPF values of 15 or more satisfy the (new) broad
spectrum standard (including the new ratio requiring proportionate protection), this proposal will
also enable consumers to select a product primarily by numerical (SPF) value on the label,
having assurance that, when used as directed, a product labeled with a higher numerical SPF
value provides proportionately more protection not only against sunburn, but also against skin
cancer and skin aging than lower numbered products
62
(provided that the product provides an
SPF of at least 15). In doing so, this proposal also eliminates another source of potential
confusion permitted by the current labeling regime, in which a higher numbered product (for
example, one labeled SPF 30) may provide inferior protection against UVA radiation than a
lower numbered product (for example, one labeled Broad Spectrum SPF 15).
III. Sunscreen products with SPFs < 15
As noted above, sunscreen products with SPF values below 15 have not been shown to
reduce the risk of skin cancer or early skin aging caused by the sun, whether or not they provide
broad spectrum protection. Because of this limitation, we considered proposing to remove from
the monograph sunscreen products with SPF values lower than 15. However, as the Surgeon
General has acknowledged (U.S. Department of Health and Human Services, n.d.), some
consumers may seek intentional sun exposure because (for example) they associate tanned skin
with attractiveness and health. These consumers may seek some protection from sunburns and
therefore, select a low SPF product (i.e., one with an SPF value below 15). If such products are
removed from the market, these consumers may choose not to use a sunscreen product at all
rather than use a broad spectrum product with an SPF of 15 or above.
Although the benefits of sunscreen products with SPFs below 15 (which are not indicated
to reduce the risk of skin cancer or early skin aging) are limited, FDA believes that the use of
such products is preferable to the use of no sunscreen at all. Thus, to provide sunburn protection
for these consumers, FDA is proposing that sunscreens with SPF 2 to 14 that bear prominent
62
As noted in section III.A.2, only those broad spectrum sunscreen products that have an SPF of 15 or higher have
been shown to help prevent skin cancer and early skin aging.
Proposed Order OTC000008
Page 73
labeling regarding their limited use for sunburn prevention and the risks associated with
spending time in the sun (see sections VI.E.ii.1 and VI.E.iii) may remain on the market without
approved NDAs under section 505G(b)(1)(A) of the FD&C Act. Because products with SPFs
below 15 have not been demonstrated to reduce the risk of skin cancer, FDA is not proposing to
require products with SPF values under 15 to pass the broad spectrum test. However, we seek
comment on whether the limited benefits such sunscreen products confer outweigh the risks of
sunscreen drug exposure and the potential false sense of security provided regarding UV
protection (i.e., whether such sunburn-only sunscreen products are GRASE and should remain
on the market without approved NDAs).
4. Maximum SPF Value Proposals
I. Maximum labeled SPF value would be SPF
60+
In conjunction with the broad spectrum proposals described above, we are also proposing
to establish a maximum labeled SPF value for products containing sunscreen monograph active
ingredients of SPF 60+. Under this proposal, sunscreen products with SPF values of 60 or
greater would be labeled “SPF 60+.”
FDA has addressed the maximum SPF value that sunscreens marketed pursuant to the
OTC Monograph System can display on their labeling several times. In the 1978 notice of
proposed rulemaking, we proposed that such sunscreens be labeled with a maximum SPF value
of 15 (43 FR 38206 at 38213 to 38214). In the 1999 final monograph, we determined that that
cap should be increased to SPF 30+ (64 FR 27666 at 27675). In 2007 (72 FR 49070 at 49085 to
49087) and then in 2011 (2011 Max SPF PR), we tentatively concluded that data existed to show
that sunscreens with labeled SPF values of up to 50+ provide additional clinical benefit to
consumers. Our proposal today to set the maximum labeled SPF value at 60+ is similarly based
on data showing the additional clinical benefit provided by SPF 60 sunscreen products when
those products also provide broad spectrum protection.
In the 2011 Max SPF PR proposing an SPF 50+ cap, we noted that the record, at that
time, lacked adequate data demonstrating that sunscreen products with SPF values above 50
provided additional meaningful clinical benefit over and above what was provided by SPF 50
protection (76 FR 35672 at 35672 to 35674). We requested data showing that such clinical
benefits existed (id.). In response to both the 2007 and 2011 proposals, we received comments
providing citations to data showing the additional meaningful clinical benefit provided by
sunscreen products with SPF values of 60 for certain at-risk populations when those sunscreens
also included broad spectrum protection. (See, e.g., Ulrich et al. (showing statistically
significant protection of organ transplant recipients, who are highly susceptible to nonmelanoma
skin cancer, from squamous cell carcinoma with use of broad spectrum SPF 60 sunscreen)

Proposed Order OTC000008
Page 74
(Ulrich et al. 2009); see also Comment FDA-1978-N-0018-0710, August 31, 2011, citing Kuhn
et al. (showing statistically significant prevention of skin lesions in topical lupus erythematosus
patients with use of broad spectrum SPF 60 sunscreen after exposure to either UVA I source or
UVA II/UVB source) (Kuhn et al. 2011); Faurschou et al. (showing prevention of urticarial
reaction in subjects with idiopathic solar urticaria with use of broad spectrum SPF 60 sunscreen)
(Faurschou and Wulf 2008); Fourtanier et al. (showing lower levels of polymorphous light
eruption in subjects using broad spectrum SPF 60 versus SPF 50 products (Fourtanier et al.
2008)). Based on the additional meaningful clinical benefit provided by broad spectrum SPF 60
sunscreens shown in these studies, we are proposing that the maximum labeled SPF value should
be SPF 60+.
Because the studies demonstrating the additional meaningful clinical benefit provided by
SPF 60 sunscreens all used sunscreens that also provided broad spectrum protection, however,
the additional clinical benefit shown to exist at SPF 60 cannot be decoupled from the broad
spectrum protection provided by those products. That is, the additional meaningful clinical
benefit shown in these studies may have been the result of the sunscreens’ protection against rays
in the UVB range or in the UVA range, or both. For this reason, our proposal to recognize the
additional meaningful clinical benefit provided by sunscreens with SPF values above 50 is
consistent with, and dependent upon, our proposal that all sunscreen products with SPF values of
15 and above be required to provide broad spectrum protection.
Given the lack of data showing that sunscreens with SPF values above 60 provide
additional meaningful clinical benefit, however, we are proposing not to allow labeled SPF
values higher than 60+ under the monograph (see section 505G(b)(1)(C) of the FD&C Act).
Labeling sunscreen products with SPF values higher than what has been shown to provide
additional meaningful clinical benefit could have unintended negative consequences. For
example, as discussed above, such products may inadvertently promote extended solar exposures
because consumers feel protected and assume that the higher SPF value implies that greater UV
exposure is safe (see, e.g., Autier et al. 2007).
II. Formulation cap for sunscreen products of
SPF 80
Although we are proposing that the maximum labeled SPF value will be SPF 60+, we are
proposing (under section 505G(b)(1)(A) of the FD&C Act) to permit the marketing of sunscreen
products formulated with determined
63
SPF values up to 80. We are proposing to permit this
additional formulation margin in part because of the inherent variability in SPF test results. A
63
As used in this proposed order, the determined SPF value is the SPF value that equals the largest whole number
less than SPF
(
t SE
)
, determined for a sunscreen product in accordance with proposed § M020.80. We propose
to define this term in § M020.3 of the monograph.

Proposed Order OTC000008
Page 75
sunscreen product’s SPF value is calculated from measurements that are based on an
investigator’s visual evaluation of an individual test subject’s erythema response to a series of
UV doses administered in successive sites on the subject’s back. Because the administered UV
dose series for the final minimal erythema dose (MED)
64
of a sunscreen with an expected SPF of
60 increases by 15 percent with each successive dose (see Deemed Final Order § M020.80(e)(3);
§ M020.80(f)(3) of this proposal), a difference in judgment of one site in opposing directions
would result in up to approximately 30 percent variability in the assessment of the amount of
exposure that resulted in the erythema.
65
Allowing the marketing of sunscreen monograph products with determined SPF test
results up to 80 would, therefore, more fully account for the range of variability in SPF test
results for sunscreen products labeled SPF 60+. We are also proposing this formulation margin
to provide additional formulation flexibility that we hope will help facilitate the development of
products with greater UVA protection, given our expectation that active ingredients added for the
primary purpose of increasing UVA protection would contribute to a sunscreen’s determined
SPF value as well. We seek comment on whether SPF 80 is the appropriate formulation cap to
accomplish these objectives.
We are proposing not to allow the marketing (without an approved NDA) of sunscreen
products with determined SPF values above SPF 80. This proposal follows from the principle
that if the addition of ingredients to a drug does not provide additional clinical benefit but
potentially increases the risk associated with the drug, this shifts the benefit-risk calculation and
renders the drug not GRASE (see, e.g., 76 FR 35673 at 35675). In light of this principle, we
solicited comments in 2011 on the appropriateness of a formulation cap for sunscreen products
(id.).
Some of the comments that we received in response to the 2011 Max SPF PR expressed
concerns (in general) about the safety of unnecessary exposure to sunscreen active ingredients.
We received only one comment, however, directly addressing the question of an SPF
formulation cap. That comment emphasized that there was no formulation limit in other
countries using an SPF labeling cap, and that the list of permitted active ingredients in the
monograph itself establishes an SPF ceiling for the formulation as a whole. FDA rejects the
premise that the list of permitted active ingredients establishes an adequate SPF cap for
64
The minimal erythema dose (MED) is the smallest UV dose that produces perceptible redness of the skin
(erythema) with clearly defined borders at 16 to 24 hours after UV exposure (Deemed Final Order § M020.80(e)(1);
see also § M020.80(f)(1) of this proposal).
65
The determination of SPF for each subject is calculated via a ratio of the MED of protected skin over the final
MED of unprotected skin. In a scenario in which the final MED of unprotected skin is underestimated by 15 percent
and the MED of protected skin is overestimated by 15 percent, this would present approximately 30 percent
variability for the individual subject.

Proposed Order OTC000008
Page 76
sunscreen formulations, as this theory does not take into account the potential addition of new
GRASE ingredients to the list of active ingredients under the monograph. This comment also
appears to imply that the maximum concentration of each active ingredient correlates specifically
to a particular numerical contribution to the total SPF value of the product. This has not been
established (see 64 FR 27666 at 27674 and 27675 (noting that formulation techniques may
enable increases in SPF without use of higher concentrations of active ingredients)). In addition,
as mentioned in the 2011 Max SPF PR (76 FR 35672 at 35674), the theoretical increase in
protection implied by higher SPF values generated in a laboratory does not necessarily
correspond to meaningful additional sunburn protection for consumers in actual use conditions.
Given that a solar simulator in a lab can produce much higher UV doses than a consumer would
receive from the sun (even in the most extreme situations), it is unlikely that a consumer could
ever actually reach the theoretical ceiling created by the list of permitted active ingredients.
Given the lack of demonstrated clinical benefit for sunscreens with determined SPF
values above SPF 60, and the potential for risks—discussed elsewhere in this document—
associated with exposure to sunscreen active ingredients, we propose not to permit the marketing
(without an approved NDA) of sunscreen products with determined SPF values above SPF 80
(see section 505G(b)(1)(C) of the FD&C Act). This proposal reflects a formulation margin
intended both to give full effect to the SPF 60 limit and to enable formulation flexibility.
III. Proposal for ≥ SPF 15 labeling
Finally, we are proposing to require that sunscreen monograph products with determined
SPF values of 15 or above be labeled with an SPF number corresponding to the lowest number in
a range of tested SPF results, as shown in table 5.
66
For example, sunscreens testing at SPF 15 to
19 would be labeled “SPF 15”; those testing at 40 to 49 would be labeled “SPF 40.”
67
This proposal is designed to avoid misleading consumers about the relative efficacy of
sunscreen products, given the lack of clinical data showing meaningful efficacy differences
between closely grouped SPF values. We note that in the 2011 L&E Final Rule, FDA declined a
request that SPF be labeled in multiples of five, stating that there was no mathematical or
statistical basis for this labeling approach because SPF values could generally be determined
with a precision that allowed for SPF values to be labeled in intervals of less than five units.
New data showing variability both between tested SPF values for individual study subjects and
for determined SPF results achieved across multiple labs testing the same sunscreen formulation
66
We note that the use of ranges to represent SPF values on product labeling is already in use in Australia and the
European Union (Therapeutic Goods Administration 2016; European Commission 2006).
67
The proposed labeled values are expressed in increments of 5 for products with determined SPF results of 15 to
29.9 (i.e., SPF 15, SPF 20, SPF 25), but for determined SPF results of 30 or more, the proposed labeled values are
expressed in increments of 10 (i.e., SPF 30, SPF 40, SPF 50, with a proposed maximum of SPF 60+.).
Proposed Order OTC000008
Page 77
(i.e., variability inherent in a clinical test that relies on visual assessments) (FDA-1978-N-0018-
0740, 2011; Stanfield et al. 2011), however, has caused us to reexamine this issue.
As described above, the clinical SPF test is conducted using a solar simulator to
administer several specified doses of UV radiation that increase by 15 to 25 percent with each
successive dose to a human subject’s back in both sunscreen-treated and untreated areas (with
the specific UV doses being derived from the expected SPF of the product and a determination of
the individual subject’s UV sensitivity). The clinical investigator then visually evaluates both
the sunscreen-treated and untreated areas of the subject’s back to identify the areas with
perceptible skin redness (erythema) that has clearly defined borders. Determining which of
several areas on a single subject’s back should be considered to meet this “clearly defined
borders” criteria is an exercise of clinical judgment. Once the investigator has made this
judgment, he or she then records the smallest dose of UV radiation it took to create an area with
the observed skin reaction of erythema with clearly defined borders. After assessing multiple
individual test subjects this way, the resulting UV exposure information is used in calculating the
determined SPF value of the sunscreen being tested. The data we reviewed suggest that the
clinical evaluation undertaken during this process creates variability that justifies the use of SPF
ranges.
For example, in a study using panels of five subjects, the mean SPF values observed
across multiple labs ranged from 54 to 82 for a target SPF 80 (FDA-1978-N-0018-0740, 2011).
This same study also evaluated a scenario where a lab was not told the target SPF, but was rather
given a range of SPF 20 to 100 for a product with an expected SPF of 100. The results showed
that it was extremely difficult for labs to reproduce the labeled SPF 100, with mean SPF values
ranging from 37 to 75. In a second study with multiple panels of 25 subjects that was controlled
and randomized, the determined SPF of two sunscreen formulations tested across four labs
ranged from 63 to 69 for a target SPF 70 and from 82 to 89 for a target SPF 90 (Stanfield et al.
2011). Although the magnitude of the differences observed in this second study were not
statistically significant, the fact that multiple labs determined different specific numerical values
for a single formulation suggests that the use of labeled values representing ranges more
accurately represents the sun protection provided by a product, and therefore is appropriate to
avoid misleading consumers.
We note that variability in SPF values is exacerbated at high SPFs. For example,
individual test results with 30 percent variability from a determined SPF value of 20 would range
from SPF 14 to SPF 26; individual test results with 30 percent variability from a determined SPF
value of 50 would range from SPF 35 to SPF 65. Accordingly, as shown in table 5, we propose
that the range of tested values reflected in the labeled SPF number should be wider at higher SPF
values and narrower at lower ones, and that the requirement that labeled SPF values correspond
to ranges rather than precise numerical values is not necessary below SPF 15.
Proposed Order OTC000008
Page 78
Table 5.--Proposed SPF Labeling Ranges
Range of Determined SPF
Values
Associated Labeled SPF
Value
60-80
60+
50-59
50
40-49
40
30-39
30
25-29
25
20-24
20
15-19
15
2-14
Determined SPF Value
iii. Proposed PDP Labeling Requirements
We are also proposing some revisions to the principal display panel (PDP) for sunscreen
monograph products (the PDP is the portion of an OTC drug product label that is most evident
when the product is displayed for retail sale (21 CFR 201.60)). In addition to satisfying general
OTC drug labeling requirements found in part 201(21 CFR part 201), sunscreen product PDPs
are currently required to satisfy specific labeling requirements located in § M020.50 of the
Deemed Final Order. We are proposing to revise the requirements for sunscreen PDP labeling
including those that address the statement of identity. We are proposing similar harmonizing
changes for the statement of identity of products that also include skin protectants. These
proposals are intended to help consumers better understand, evaluate, and compare sunscreen
products by providing additional information on the PDP, and by ensuring the prominence and
readability of information required to appear on the front of the container or package.
We are proposing to revise the SOI, which is required to appear on the PDP by the
Deemed Final Order at § M020.50(b) and would remain part of the PDP under this proposed
order. Currently, the SOI for sunscreens under the Deemed Final Order contains “the established
name of the drug, if any” and identifies the product as a “sunscreen.” The revised SOI in §
MO20.50(a)(1)(i) of this proposed order would consist of the established name of all active
ingredients in alphabetical order followed by “Sunscreen” and the product’s dosage form (such
as lotion or spray). In light of these proposals for the SOI for sunscreens, we are also proposing
similar provisions to address the SOI for products that contain both sunscreen and skin protectant
active ingredients, in both the sunscreen monograph (proposed § M020.60) and the skin
protectant monograph (proposed § M016.60). Additional proposals for monograph conditions
applicable to products that contain both sunscreen active ingredients and skin protectant active
ingredients are described in section VI.E.vi.1 of this document.
Proposed Order OTC000008
Page 79
The proposal to list all active ingredients as part of the SOI is generally consistent with
SOI labeling of other OTC and prescription drugs. Providing information about a product’s
active ingredients and dosage form would supplement other important elements of the PDP (SPF,
broad spectrum, and water resistance information) to provide a succinct summary of the
product’s key characteristics on the front of the package or container. We expect that this
approach would enable consumers to more readily compare differing products and either select
or avoid a given product accordingly. As an indication that consumers value information about a
sunscreen’s active ingredients, an analysis of top-rated sunscreen product reviews on
Amazon.com found that product ingredients were listed as a positive factor in 17 percent of
responses, and a negative factor in 10 percent of responses (Xu et al. 2016).
Based on a review of marketed sunscreen product labels, FDA is concerned that the SOI
may currently be obscured by the inclusion and prominence of other printed or graphic
information on the PDP. For this reason, we also propose to require the SOI to appear in direct
conjunction with the most prominent display of the proprietary name, in a boldface font at least
one-fourth the size of the most prominent printed matter on the PDP, and displayed so that the
text is generally parallel to the base of the packaging. We propose that the entire SOI appear in
the same font style, size, and color with the same background color, and as continuous text with
no intervening text or graphic material other than text provided in accordance with the
requirements for the SOI for a product that also includes a skin protectant, where applicable.
These requirements would supplement, and not replace, the general requirements regarding the
PDP and SOI for all nonprescription products codified in 21 CFR 201.60 and 201.61.
Section M020.50 of this proposed order would incorporate the “Broad Spectrum SPF,”
“SPF,” and “Water Resistant” statements that already must appear on the PDP as described in the
Deemed Final Order at § M020.50(a). Additionally, for all products with SPF values below 15,
we propose to require that the SPF statement be followed by an asterisk (*) directing the
consumer to the statement “*See Skin Cancer/Skin Aging Alert.” We propose that the quoted
statement must appear in the bottom 30 percent of the PDP. This statement is intended to draw
the consumer’s attention to the Skin Cancer/Skin Aging Alert that would continue to be required
for these products as part of the “Warnings” in the Drug Facts portion of the label (§ M020.52(b)
of this proposed order and § M020.50(d) of the Deemed Final Order), because there is evidence
that some sunscreen consumers are not reading this information in its current location (Chao et
al. 2017; Kong et al. 2015).
Under the Deemed Final Order, the entirety of the “Broad Spectrum SPF” or “SPF”
statement, as applicable, must appear on the sunscreen PDP in the same font style, size, and color
and with the same background color, and, if used, the “Broad Spectrum SPF” statement must
also appear as continuous text with no intervening text or graphic. To further ensure the
prominence and readability of information that is important for consumers to evaluate and
Proposed Order OTC000008
Page 80
compare sunscreen products, we propose to retain these requirements and to specify that these
statements must also appear in bold typeface at least one-fourth the size of the most prominent
printed matter on the PDP, and as text generally parallel to the base of the packaging.
The proposed new “*See Skin Cancer/Skin Aging Alert” statement would also be
required to appear in bold typeface at least one-fourth the size of the most prominent printed
matter on the PDP, and as text generally parallel to the base of the packaging. In addition, the
entire statement would appear in the same font style, size, and color with the same background
color, and as continuous text with no intervening text or graphic.
Finally, because water resistance is also an important characteristic for consumers when
choosing a sunscreen, we also propose to apply comparable format requirements to the “Water
Resistant” statement. The statement would also be required to appear in bold typeface at least
one-fourth the size of the most prominent printed matter on the PDP, and displayed so that the
text is generally parallel to the base of the packaging. In addition, the entire statement would
appear in the same font style, size, and color with the same background color, and as continuous
text with no intervening text or graphic (see proposed § M020.50(a)(3)).
iv. Proposed Requirements Related to Final Formulation Testing
and Recordkeeping
We are also proposing a number of provisions (1) to ensure that efficacy testing of the
sunscreen formulation to be marketed is conducted in a way that protects human subjects and
produces reliable results and (2) to enable FDA to assess compliance with final monograph
conditions going forward.
1. General Approach to Final Formulation Testing
The Deemed Final Order currently includes technical instructions for conducting the final
formulation testing required to support the SPF values, water resistance statements, and broad
spectrum statements shown in sunscreen product labeling. However, the Deemed Final Order
does not explicitly address important broader considerations that are essential to ensuring that
final formulation testing is conducted and documented in a way that verifiably provides for
protection of human subjects in SPF and water resistance testing, as well as ensuring the
reliability of all the testing data that underlies marketing in accordance with monograph
conditions. We expect that persons responsible for conducting final formulation testing should
already be following best practices in their current testing programs. However, we are concerned
that many entities may not uniformly observe such practices and/or may not maintain the records
needed to document compliance with the final formulation testing procedures set forth in the
Deemed Final Order. FDA’s experience in conducting inspections and other actions to verify
testing under the operative requirements for nonprescription sunscreens marketed without an
Proposed Order OTC000008
Page 81
NDA have suggested latent problems in these areas. Although limited, this experience reinforces
FDA’s belief that further clarification of Agency expectations is necessary given the public
health importance of ensuring that sunscreen products are safe and effective under the conditions
of use set forth in the monograph, and the broad range of entities that may be involved in
bringing sunscreen products to market. Thus, we are proposing to incorporate FDA’s current
expectations more explicitly in the proposed order. The proposed provisions are broadly
consistent with current best practices for efficacy testing conducted in human subjects and are
not expected to require significant changes by reputable and experienced testing establishments.
Key areas of concern that are addressed by the proposals include the following.
I. Protection of human subjects and oversight of
clinical final formulation testing
Ensuring that clinical final formulation testing is both designed and conducted in a
manner that will yield reliable results is critical, as is ensuring the protection of the human
subjects on whom SPF and water resistance testing are conducted. Existing provisions within
the SPF test in § M020.80 of the Deemed Final Order require that informed consent be obtained,
but do not otherwise specify what this should involve or how clinical final formulation testing
should be overseen. Across disciplines, testing involving human subjects is ordinarily conducted
under institutional review board (IRB) oversight as a means of ensuring that informed consent
and other human subject protections are provided and ensuring the integrity of study design and
execution. FDA likewise expects that IRB review is already routinely being obtained by many
establishments for SPF and water resistance testing.
Nonetheless, our experience in conducting inspections and other actions to verify the
reliability of final formulation testing under the current requirements for nonprescription
sunscreens marketed without an NDA have raised some questions about current practices. For
example, FDA’s observations have raised questions about whether and how entities conducting
final formulation testing have put in place protocols and IRB oversight to ensure that test
subjects do not repeat participation in testing with a frequency that could both compromise the
ability to distinguish erythemic reactions to the test article and raise other questions about human
subject protection. We are concerned that the lack of explicit requirements with regard to IRB
oversight, as well as the cursory nature of the informed consent requirement in the current
labeling and testing requirements for nonprescription sunscreens marketed without an NDA, may
result in inconsistent practices in the conduct of SPF testing that would compromise the
reliability of results. Among other things, IRB review is critical to verify the adequacy of
informed consent and to ensure that study protocols incorporate appropriate inclusion/exclusion
criteria for subject selection (both to protect test subjects and to ensure the accuracy of results).
II. Qualifications of study personnel
Proposed Order OTC000008
Page 82
In some instances, it may not be clear upon inspection whether all aspects of a study were
conducted by appropriately qualified personnel. For example, FDA would not consider it
adequate for a technician, rather than an appropriately trained medical professional (such as, for
example, a nurse or dermatologist), to perform a physical examination for potential nevi, moles,
or other dermal lesions. As with all clinical and nonclinical testing done to support labeling and
marketing, the use of properly trained and appropriately qualified personnel is essential to ensure
the reliability and accuracy of test results. Documentation of the qualifications and training of
personnel is also necessary to enable FDA’s ability to assess compliance with monograph
conditions.
III. Documentation of equipment maintenance,
study methods, and observations.
Failure to maintain adequate records of testing equipment, methods, and observations can
raise broad questions about the reliability of final formulation testing. In FDA’s experience
since the promulgation of 21 CFR 201.327 (now incorporated in the Deemed Final Order), there
has been a lack of uniformity in testing entities’ approaches to recordkeeping for final
formulation testing, raising concerns about the adequacy of recordkeeping procedures. Failure of
testing entities to keep adequate records to support final formulation testing may leave FDA
unable to verify that the UV doses provided in SPF and water resistance test reports are accurate
and valid. This is also true with respect to documentation of emission spectrum, the percentage
of erythema-effective radiation contribution, and changes to solar simulator components and the
UV meter/dose controller system. Failure to accurately calibrate and maintain equipment at one
testing entity may affect data across multiple clinical SPF testing studies and/or broad spectrum
testing for multiple different final formulations that are ultimately sold under different labels.
We propose to address these concerns and align the OTC monograph for sunscreens with our
existing expectations through revised conditions of marketing that are described further in the
following sections.
2. Specific Proposals
I. Consequences of failure to observe best
practices
Unless testing is conducted in compliance with all applicable provisions of the proposed
order, FDA will not have adequate assurance that the labeling reliably reflects the properties of
the sunscreen product. Therefore, if final formulation testing is not properly conducted in
accordance with these conditions, labeling a sunscreen with an SPF value or representation of
water resistance or broad spectrum properties based on that testing is a misrepresentation to the
consumer that the labeling reliably states the product’s properties, which should also be
consistent with a system of standardized sunscreen labeling that can be used to make cross-
product comparisons. If these provisions are finalized, failure to comply with these conditions
Proposed Order OTC000008
Page 83
would make a drug subject to regulatory action as misbranded under section 502(ee) of the
FD&C Act (21 U.S.C. 352(ee)) and an unapproved new drug.
II. General obligations of responsible persons
We are aware that many different business relationships involving numerous entities are
commonly used in the manufacturing, testing, and labeling of nonprescription sunscreen drug
products. To clarify the locus of responsibility for ensuring that adequate final formulation
testing procedures are in place, and to clearly delineate responsibility for recordkeeping related
to final formulation testing, FDA proposes a new defined term, responsible person.
We propose to define the term responsible person in a way that is consistent with FDA’s
treatment of regulatory responsibilities for other OTC drug products and that is in alignment with
requirements for adverse event reporting for OTC drug products, in section 760(b)(1) of the
FD&C Act (21 U.S.C. 379aa(b)(1)). The proposed definition for responsible person is “the
manufacturer, packer, or distributor whose name appears on the labeling of a sunscreen product
covered by this OTC monograph.” Defining responsible person in this way will enable FDA to
better assess compliance with OTC monograph conditions because it creates a chain of
responsibility that is immediately apparent from the product’s labeling. The responsible person,
as identified on the labeling, is ultimately responsible for ensuring that the product bearing its
name is labeled in accordance with the requirements of the OTC monograph.
The proposed provisions would broadly set forth the general obligations of responsible
persons with respect to final formulation testing for nonprescription sunscreens marketed without
an NDA, and they would make clear that the responsible person is charged with ensuring that
sunscreen products are appropriately tested. The obligations of responsible persons as set forth
in this proposed order are modeled after those of investigational new drug application (IND)
sponsors under part 312 (21 CFR part 312), but are somewhat modified to accommodate unique
aspects of clinical and nonclinical sunscreen final formulation testing. Because final formulation
testing for nonprescription sunscreens marketed without an NDA is intended to verify the
claimed properties of a final formulation, and because this purpose is narrower in scope and
duration than most clinical testing performed under FDA’s IND regulations in part 312, a
responsible person under this proposed order would have responsibilities that incorporate some
of the traditional responsibilities of investigators as well as those of sponsors under part 312. For
example, FDA proposes to clarify that responsible persons must select appropriately qualified
personnel to conduct testing, ensure compliance with the requirements for IRB review and
obtaining informed consent, and monitor the compliance of personnel with investigators’
statements.
Proposed Order OTC000008
Page 84
This proposed approach accounts for situations in which investigators and other
personnel conducting final formulation testing are employees of the responsible person. We also
propose to clarify that the responsible person must ensure that investigators and other personnel
conducting SPF testing for a nonprescription sunscreen marketed under the OTC monograph
comply with requirements related to human subject protection and the appropriate conduct of
clinical testing. We believe that this better reflects the employer/employee relationships that are
more common in connection with final formulation testing rather than with clinical testing
conducted under an IND. These proposed provisions regarding selection of personnel are also
consistent with the existing obligations of manufacturers under parts 210 and 211 (21 CFR parts
210 and 211), both of which govern compliance with CGMPs.
The proposals in this order would also permit a responsible person to transfer some or all
of its obligations to another entity, consistent with current industry practice, except for
obligations with respect to recordkeeping. The recordkeeping proposal is discussed in section
VI.E.iv.2.VIII of this document. Failure of an entity to comply with monograph conditions
governing responsibilities it has assumed would subject that entity to the same regulatory action
as if it were a responsible person who had failed to comply with those obligations. This
provision is analogous to the provision in FDA’s regulations in part 312 allowing for transfer of
obligations of IND sponsors.
III. Adequate clinical testing procedures and
conditions
Although the Deemed Final Order requires “legally effective written informed consent
from all test subjects” (§ M020.80(c)(4)), it does not address broader underlying requirements
for conducting clinical testing. In light of the concerns we identified regarding current clinical
testing procedures and conditions, we propose to address adequate clinical testing procedures
and conditions in the OTC monograph. We expect that final formulation testing conducted in
compliance with the proposals in this proposed order will be more likely to ensure protection of
human subjects while also more reliably determining the SPF value and water resistance
properties of the final formulations being tested. Unless appropriate clinical testing procedures
and conditions are adhered to, FDA cannot have confidence in the resulting labeled SPF and
water resistance properties of the product.
The proposed order would also include provisions to make clear that FDA’s regulations
governing informed consent (21 CFR part 50) and IRB approval of research (21 CFR part 56)
apply to clinical final formulation testing that is conducted to support marketing of sunscreens
under the OTC monograph. In our view, as a matter of good clinical practice, IRB approval
should already be routinely, currently obtained for clinical final formulation testing under the
Deemed Final Order because it is essential to producing results that are scientifically sound and
ethically appropriate. Because clinical final formulation testing required to support the GRASE

Proposed Order OTC000008
Page 85
status of nonprescription sunscreens marketed without an NDA is not conducted under an IND or
in support of the inclusion of an ingredient as a GRASE condition of marketing under the
procedures set out in 21 CFR part 330,
68
it was not previously included explicitly in the scope of
testing covered by 21 CFR parts 50 and 56. We propose to rectify this omission by explicitly
cross-referencing parts 50 and 56 in the OTC monograph conditions for final formulation testing.
This will clarify that both of these parts apply to clinical final formulation testing for sunscreens
and will resolve any inconsistency in current practice.
The proposed reference to 21 CFR part 50 clarifies FDA’s position that legally effective
written informed consent to participate in clinical final formulation testing should share the same
properties as informed consent required for all other clinical testing covered by FDA’s
regulations in that part. Similarly, by referencing 21 CFR part 56, the proposal ensures that final
formulation testing is held to the same standards for IRB review as other clinical testing covered
by FDA’s regulations. In reviewing clinical protocols, IRBs have the ability to determine
whether the protocol is adequately designed to study the endpoints sought, and to ensure that
protocol elements, such as enrollment criteria, adequately protect both human subjects and the
scientific rigor of the experiment.
IV. Control of personnel
We propose to place responsibility on the responsible person to ensure that investigators
and other personnel conducting clinical final formulation testing adhere to the investigational
plan, the signed investigator statement, and all applicable orders and regulations. We also
propose to place responsibility on the responsible person for ensuring human subjects’
protection, including through appropriately reporting changes in the testing to IRBs, and by
appropriately seeking prior IRB approval for any changes to the testing, except where necessary
to eliminate apparent immediate hazards to human subjects. Under the proposed order,
responsible persons are also expected to obtain from each investigator, and retain for their
records, a signed investigator statement. This requirement is similar to what is required of
sponsors of INDs, and it helps to ensure that the investigator is qualified, understands his or her
obligations, and will comply with the applicable requirements of this OTC monograph and with
the protocol. It also enables better oversight of clinical investigations by FDA because it creates
a record of the investigator’s relevant experience and qualifications.
68
We note that certain parts of 21 CFR part 330 have been affected by enactment of section 505G of the FD&C Act,
and that appropriate regulatory changes (to withdraw certain parts of the regulation and make corresponding
technical changes) are forthcoming. See section 505G(k)(3) of the FD&C Act.
Proposed Order OTC000008
Page 86
V. Research monitoring
The proposed order also includes a number of proposals to ensure adequate monitoring of
clinical final formulation testing. The proposed provisions would require that responsible
persons inform all investigators testing a formulation if there are new observations about the
drug, particularly with regard to adverse events or safe use. These proposed requirements are
necessary to ensure proper communication between study personnel and protection of human
subjects. Responsible persons must also monitor the conduct of investigations to ensure that
clinical testing is being conducted in accordance with the protocol and with applicable OTC
monograph conditions and regulations. If a responsible person discovers noncompliance by
study personnel, then the responsible person must either secure compliance or remove the
noncompliant personnel from conducting testing.
Finally, we propose to require that investigators report adverse events and/or safety
concerns to the responsible person, and that investigators also provide responsible persons with
final reports at the conclusion of testing. We believe that this proposed requirement will ensure
there is appropriate documentation and communication of adverse events and/or safety concerns
that arise during testing. It will also ensure there is a record of SPF testing conducted for
nonprescription sunscreens marketed without an NDA that can be relied upon should questions
related to a particular formulation arise when the sunscreen formulation is marketed. The
proposed requirements are consistent with reporting required in the IND context, although—
because of the short duration of the clinical final formulation testing conducted for
nonprescription sunscreens marketed without an NDA-- we are not proposing to require annual
reporting.
VI. Test subject selection
We also propose language regarding the selection of test subjects for final formulation
testing conducted to support marketing of nonprescription sunscreens under the OTC
monograph. This is an area in which FDA’s inspections of testing entities have suggested a lack
of consistency. We are particularly concerned that inclusion/exclusion criteria provide for
adequate time between study and enrollment and prior UV exposure, such as from participation
in a previous SPF test, sunbathing, or sunlamp use. Erythemal responses can remain for days
after sunbathing, and it is known that pigmentation development takes up to a week after initial
exposure and remains for weeks to months (Coelho et al. 2009). SPF clinical studies should not
include individuals who have participated in sunbathing, tanning bed use, or another SPF clinical
study for at least the past 4 weeks or perhaps longer if UV-induced responses remain. The
proposed clarification regarding conduct of physical examinations of test subjects reflects this
consideration, and our additional proposal for IRB review, addressed elsewhere, will help ensure
it is appropriately acted on.
Proposed Order OTC000008
Page 87
VII. Applicability of registration and CGMP requirements
The proposed order also clarifies FDA’s existing view that final formulation testing for
nonprescription sunscreens conducted under the monograph constitutes the “manufacture” of a
drug for purposes of the application of FDA’s CGMP regulations. As such, this testing must be
conducted in an establishment registered in accordance with 21 CFR part 207 and section 510 of
the FD&C Act (21 U.S.C. 360). This interpretation is consistent with the definition of
manufacture in 21 CFR part 207, which includes “each step in the manufacture, preparation,
propagation, compounding, or processing of a drug ….” (21 CFR 207.1). The definition of
manufacture as used in part 207 also “includes manipulation, sampling, testing, or control
procedures applied to the final product or to any part of the process, including, for example,
analytical testing of drugs for another registered establishment’s drug” (id). This interpretation
is also consistent with FDA’s regulations in 21 CFR 330.1, which require that OTC monograph
drug products be manufactured in a registered establishment in order to be generally recognized
as safe and effective and not misbranded. The incorporation of this provision in the proposed
order, therefore, is intended to clarify an existing requirement for facilities performing this type
of testing.
We also propose to clarify that, as a manufacturing activity, final formulation testing
conducted under this paragraph is expected to be done in accordance with CGMPs as set forth in
21 CFR parts 210 and 211 (see 21 CFR 210.3(b)(12), indicating that for the purposes of 21 CFR
parts 210 and 211, “Manufacture, processing, packing or holding of a drug product includes
packaging and labeling operations, testing, and quality control of drug products”). This is
consistent with FDA’s regulations in 21 CFR 330.1, which require compliance with CGMPs as a
condition for OTC drug products to be GRASE and not misbranded when otherwise marketed
consistent with conditions in a final monograph. Adherence to CGMP requirements in 21 CFR
parts 210 and 211 includes compliance with the requirements to keep certain records and to have
appropriately trained and qualified personnel.
VIII. Recordkeeping
To enable FDA to better monitor compliance with the requirements of the OTC
monograph, we propose to include specific recordkeeping requirements for final formulation
testing. Accordingly, the proposed order at § M020.110 clarifies what records of testing
performed under this OTC monograph must be kept, by whom, and for how long. This provision
also allocates responsibility for records maintenance and specifies what records must be made
available to FDA for inspection. Recordkeeping is essential for FDA to evaluate whether
required testing of final formulations is being conducted in accordance with the OTC
monograph, and to enable the Agency to investigate postmarketing product failures or adverse
Proposed Order OTC000008
Page 88
events. Appropriate recordkeeping also enables FDA to conduct better and more efficient
inspections of entities conducting final formulation testing.
These recordkeeping requirements are in alignment with what is required for other types
of manufacturing under CGMPs as set forth in 21 CFR parts 210 and 211. The proposed
provisions are intended to clarify how, and for how long, records must be kept to substantiate
required final formulation testing. We are proposing that records of testing must be kept by the
responsible person (as newly defined in the proposed order, discussed previously), as well as by
any other entity that actually performs testing (under a transfer of obligations per § M020.70(a)
in the proposed order or otherwise). Requiring that records be kept by both the responsible
person and the testing entity (if different) will enable FDA to more easily identify records
supporting the labeling of any given final formulation even when the product is labeled with the
responsible person’s information, but testing and manufacturing was completed by a third party.
The proposed recordkeeping requirements reflect FDA’s experience in interacting with
regulated industry. By requiring that records be kept by both the responsible person and any
other entity that performs final formulation testing, the proposed order will enable FDA to better
monitor compliance with OTC monograph conditions for sunscreens marketed without an NDA
by, for example, allowing FDA to identify the source of formulation failures or apparent
inconsistencies between the product labeling and consumer experience. The proposed
recordkeeping requirements will also assist FDA when it is conducting inspections of entities
that perform final formulation testing for a number of different responsible persons and products,
as we believe is the norm in this industry. Having ready access to records reflecting the overall
conduct of final formulation testing during an inspection of such an entity is important because it
will enable FDA to identify potential systemic problems in final formulation testing that may
have an impact on the reliability of results supporting the labeling of multiple different sunscreen
products marketed by a variety of responsible persons. We note that these recordkeeping
requirements should not be understood to mandate duplicative records within the files of a single
testing entity or single responsible party. For example, if one investigator is responsible for
testing multiple final formulations, one copy of the signed investigator statement and Curriculum
Vitae (CV) would be sufficient to support all formulations tested by that investigator.
Consistent with FDA’s view that final formulation testing is manufacturing for purposes
of the application of FDA’s CGMP regulations, equipment maintenance records and other
records documenting compliance with CGMPs are expected to be maintained as required by 21
CFR parts 210 and 211. Accordingly, we clarify in the proposed order at § M020.110(a) that
records documenting proper maintenance of equipment used in final formulation testing must be
kept, consistent with existing obligations in 21 CFR 211.68. In our view, this clarification will
promote uniformity in adherence to best practices and will help ensure more accurate and
reliable labeling of sunscreen products based on final formulation testing. Additional specificity
Proposed Order OTC000008
Page 89
has been proposed here to clarify how the more general recordkeeping provisions of 21 CFR part
211 apply to final formulation testing. To provide assurance that the test results are not
compromised by faulty equipment maintenance or equipment failure, FDA proposes that testing
entities must keep documentation demonstrating that equipment used for final formulation
testing has been maintained in accordance with established written specifications. This
requirement will enable FDA to more efficiently monitor compliance. Consistent with the
Agency’s treatment of other CGMP requirements, without recordkeeping, there is no assurance
that a sunscreen drug product has the identity and strength, and meets the quality and purity
characteristics, which it purports or is represented to possess.
This proposal also elaborates on recordkeeping necessary to document compliance with the
requirements of the proposed order regarding conduct of final formulation testing. Proposed
required records for SPF testing include records that: (1) identify the facility conducting the
testing; (2) identify the equipment used; (3) identify product samples and lots; (4) characterize
the SPF standard that is used; (5) document parameters for water resistance testing; and (6)
demonstrate compliance with the provisions governing adequate clinical testing procedures and
conditions. For example, these would include documentation of IRB review, case histories for
each human subject (which must document protocol deviations or injuries), administration of the
sunscreen, and reading of test results. These proposed recordkeeping obligations are consistent
with those required of parties engaged in human subjects testing governed by FDA’s regulations
(e.g., 21 CFR part 312).
Required records of broad spectrum testing conducted under the proposed order would
include those records necessary for identifying the facility conducting the testing, providing
information associated with the sample, identifying equipment used, and documenting sunscreen
product application. These proposed requirements provide greater specificity than existing
requirements in FDA’s CGMP regulations and are expected to increase uniformity in current
practice. We propose to clarify FDA’s expectations regarding access to records that responsible
persons and other testing entities are required to keep under the proposed order. These proposed
monograph conditions are also consistent with FDA’s inspection authorities in section 704 of the
FD&C Act (21 U.S.C. 374).
IX. Minor proposed revisions to test procedures
In addition to the changes discussed in section VI.E.iv, we are proposing to update the
technical instructions for sunscreen final formulation testing in the Deemed Final Order to clarify
how the testing should be conducted. We are concerned that manufacturers conducting the SPF
test procedure may be relying on determinations of the initial minimal erythema dose of
unprotected skin (MEDu) generated too far in advance of testing the sunscreen product. The
current provisions in the Deemed Final Order address four different determinations of MED for

Proposed Order OTC000008
Page 90
each test subject: (1) an initial MED for unprotected skin (initial MEDu); (2) a final MED for
unprotected skin (final MEDu); (3) an MED for skin to which the SPF standard has been applied
(ssMEDp); and (4) an MED for skin to which the sunscreen test product has been applied
(tpMEDp). The initial MEDu is used to set the UV exposures administered to determine final
MEDu, ssMEDp, and tpMEDp (see Deemed Final Order at § M020.80(e)).
Although the Deemed Final Order requires that each of the MED values be determined
16 to 24 hours after UV exposure, it merely notes that the final MEDu, ssMEDp, and tpMEDp
are “typically determined the day following determination of the initial MEDu.” (See Deemed
Final Order at § M020.80(e)(4)). Because the skin reactivity of a test subject changes over time,
we propose to clarify that the initial MEDu of a person’s unprotected skin must be determined no
more than 1 day before the UV exposures for final MEDu, ssMEDp, and tpMEDp are
administered. We are also clarifying that to calculate the SPF value for each test subject, under
proposed § M020.80(g), it is the subject’s final MEDu that should be used.
In our review of the testing requirements for nonprescription sunscreens marketed
without an NDA, we also revisited our position on the input slit bandwidth specification in the in
vitro broad spectrum test. In the 2011 L&E Final Rule, now incorporated into the Deemed Final
Order, we modified the in vitro broad spectrum test that was proposed in the 2007 proposed rule
to change the input slit spectrometer bandwidth specification from ≤ 5 nm to ≤ 1 nm. After the
2011 final rule published, FDA received a comment from a spectrometer manufacturer arguing
that the 1 nm input slit bandwidth specification was unreasonable. The manufacturer argued that
common spectrometer models that are currently used to test sunscreens cannot comply with the ≤
1 nm input slit bandwidth specification, and those that can are more expensive, more difficult to
use, and take more time to use. The manufacturer provided data that indicate that spectrometers
with ≤ 1 nm input slit bandwidths do not produce more reliable results than spectrometers with
larger input slit bandwidths (see Comment, Docket No. FDA-2010-D-0509-0004, at
https://www.regulations.gov (accessed May 21, 2021)). In light of this submission, FDA
reassessed the input slit bandwidth parameters and concluded that ≤ 5 nm will be sufficient for
the broad spectrum procedure. Although decreasing bandwidth improves the ability to resolve
closely spaced peaks (i.e., the spectral resolution), this is not a significant consideration for in
vitro broad spectrum testing of sunscreen products because transmittance/absorbance curves for
sunscreen products are typically smooth with no individual sharp peaks. Accordingly, we
propose to require that spectrometer input slits for broad spectrum testing of nonprescription
sunscreens marketed without an NDA be set to provide a bandwidth that is ≤ 5 nm.
We are also proposing to update the monograph to correct a minor inaccuracy in the
Deemed Final Order language describing testing procedures. Specifically, § M020.80(a)(2)(iii)
in the Deemed Final Order states that “emission spectrum must be determined using a handheld
radiometer.” As written, this statement is inaccurate because a handheld radiometer cannot
Proposed Order OTC000008
Page 91
determine the emission spectrum of a solar simulator. We propose to resolve this error by
clarifying that the handheld radiometer measures the solar simulator radiation intensity rather
than the emission spectrum (see proposed § M020.80(b)(2)(iii).) Finally, we have proposed
updates to language describing final formulation testing procedures to clarify our long-standing
intention that these provisions of the test are requirements, not merely suggestions.
v. Proposed Status of Sunscreen-Insect Repellent Combination
Products
1. Background
Sunscreen-insect repellent combination drugs are products used on human skin that
contain both a sunscreen drug component and an insect repellent component. FDA is aware of a
number of such products having varying insect repellent ingredients, SPF levels, dosage forms,
and other features. FDA regulates sunscreens as drug products under the FD&C Act, and EPA
concurrently regulates insect repellents as pesticides under FIFRA.
69
FIFRA defines a
“pesticide” in relevant part as “any substance … intended for repelling … any pest,” including
insects (7 U.S.C. 136)(u)). Before they can be marketed, most skin-applied insect repellents
must be registered by EPA, although a few plant-derived insect repellent active ingredients are
exempt from registration because EPA has determined they present minimum risk potential to
humans (EPA 2015).
Sunscreen-insect repellent products have been marketed in the United States since before
the OTC review began, but were not addressed in the rulemaking for the OTC sunscreen
monograph prior to publication of the 2019 Proposed Rule (84 FR 6204 at 6244). Both FDA and
EPA had historically declined to object to the marketing of these products pending the issuance
of a final sunscreen monograph, provided that the sunscreen active ingredient(s) is listed in the
(then) stayed final monograph and the insect repellent component is registered with the EPA (79
FR 7941 at 7943). In 2011, FDA issued a draft enforcement guidance intended for
manufacturers who market OTC sunscreen products without an approved application, which
recommended that manufacturers of sunscreen-insect repellent combination products should
comply as closely as possible with FDA’s sunscreen testing and labeling requirements then
included in 21 CFR 201.327, now part of the Deemed Final Order at §§ M020.50, M020.80, and
69
Some insect repellents are also regulated by FDA as human drugs (e.g., pediculicides and scabicides intended to
control parasites on humans) or animal drugs (e.g., pesticide products for oral administration to animals) (7 U.S.C.
136 et seq.); see also “MOU 225-73-8010 Memorandum of Understanding Between the Environmental Protection
Agency and the United States Department of Health, Education and Welfare Food and Drug Administration,”
(available at
https://www.fda.gov/aboutfda/partnershipscollaborations/memorandaofunderstandingmous/domesticmous/ucm1158
73.htm (accessed May 21, 2021).
Proposed Order OTC000008
Page 92
M020.90. This guidance was finalized in May 2018 (FDA 2018a), but withdrawn (as noted
above) in September 2021 in conjunction with posting of the Deemed Final Order.
In the Federal Register of February 22, 2007 (72 FR 7941), FDA issued a notice seeking
public comments on sunscreen-insect repellent combination products, and, in particular, whether
FDA should amend the OTC sunscreen monograph to add conditions for marketing insect
repellent-sunscreen drug products (FDA Call for Data or call for data). The call for data
summarized the regulatory status and history of both sunscreens and insect repellents and sought
public input on a number of issues (see table 6). On that same date (February 22, 2007, 79 FR
7979), EPA published a similar notice announcing that it was also seeking information to
determine how insect repellent-sunscreen combination products should be regulated.
Table 6.--Key Issues and Information Requests in FDA’s 2007 Call for Data.
General Issue
Key Concerns and Information Requests
Possible manufacturing
conflicts
Requested information about whether there are known
conflicts between FDA and EPA manufacturing requirements
and, if so, how to resolve them.
Asked how FDA should address EPA-registered insect
repellents in finalizing the OTC sunscreen monograph; which
requirements should FDA retain, revise, or eliminate?
Inquired about manufacturer testing of sunscreen-insect
repellent combination products and whether any problems
were encountered.
Possible formulation conflicts
Requested comments on the significance of published
research suggesting a potential formulation conflict.
Possible labeling conflicts
between OTC sunscreen
monograph and EPA
registration requirements
70
Labeling differences noted:
•
FDA uses “warning”; EPA uses “caution” (and only
uses the word “warning” to indicate toxicity levels).
•
Many differences in required warning/caution section
headings.
•
Directions for sunscreen use call for liberal
application and frequent reapplication; EPA
directions may limit where and how to apply product
and restrict frequency of application.
70
Since publication of the call for data, FDA has established additional labeling regulations for certain OTC
sunscreen products marketed without approved applications. However, the labeling concerns expressed in the call
for data remain relevant.

Proposed Order OTC000008
Page 93
Asked whether different directions for use can be integrated
without leading to improper application, overexposure to
insect repellent, and/or underexposure to sunscreen.
FDA requires the outside container or wrapper of the retail
package or the immediate container label to list all active and
inactive ingredients (see section 502(e)(1)(A)(iii) of the
FD&C Act; 21 CFR 201.66(c)). EPA requires listing of the
percentage of each active ingredient, and the total percentage
of all “inert” or “other” ingredients, in the pesticide. Inert
ingredients are not required to be identified individually on
the product except in certain cases (in which case all inert
ingredients are listed). Asked whether there is a way to label
combination sunscreen-insect repellent drug products in a
way that satisfies both the requirements of the FD&C Act
and the FIFRA, and whether “inert” ingredients under the
FIFRA are equivalent to “inactive” ingredients under the
FD&C Act.
Safety issues
More safety data needed given published animal studies
indicating increased absorption of DEET and various
sunscreens active ingredients when the components are
combined. Asked for more safety data on combined
products.
Requested data on whether increased absorption of a
sunscreen ingredient occurs when combined with an insect
repellent.
Information needed about incidence of skin irritation from
combination products.
Effectiveness issues
Requested information on:
•
Possible effects of insect repellent on sunscreen SPF;
possible decreased sunscreen efficacy or increased
exposure to insect repellent without greater efficacy
resulting from inconsistent reapplication intervals.
•
Potential chemical or physical incompatibilities
between particular sunscreens and insect repellents.
•
Potential need to specify minimum SPF for these
combinations.

Proposed Order OTC000008
Page 94
•
Any potential performance benefits of these
combination products other than convenience.
•
Possible adjustments to formulations to minimize
application time disparities.
2. FDA’s Evaluation of Sunscreen-Insect Repellent Combination
Products
FDA has reviewed the comments submitted in response to FDA’s and EPA’s calls for
data, as well as pertinent scientific literature and publicly available EPA regulatory documents.
In evaluating combination insect repellent-sunscreen products for the purposes of this rule, FDA
defers to EPA’s expertise and authority regarding insect repellent ingredients. We have not
independently evaluated these pesticides, but instead have focused on potential sunscreen-insect
repellent ingredient interactions and the feasibility of effectively labeling these combination
products.
We have tentatively concluded that conflicting labeling requirements for the sunscreen
and insect repellent components of sunscreen-insect repellent combinations cannot be reconciled
to create labeling that will sufficiently ensure safe and effective use of the sunscreen component.
For this reason, we propose that sunscreen-insect repellent products are not GRASE for use as
sunscreens under section 201(p)(1) of the FD&C Act. See section 505G(b)(1)(C)(i) of the FD&C
Act. We also note that these conflicting requirements prevent these products from having
adequate directions for use as a sunscreen, and thus these products would be misbranded under
section 502(f) of the FD&C Act. Also, if we did not have this labeling concern, we would still
tentatively determine that these products are not GRASE for use as sunscreens because the
available evidence regarding the safety and effectiveness of these products for their use as
sunscreens are inadequate to show that they are GRASE for such use. FD&C Act section
505G(b)(1)(C)(ii). Specifically, evidence suggests that interactions between some sunscreen
active ingredients and insect repellents may decrease safety by increasing systemic absorption of
one or both components, and potential synergistic effects on the efficacy of sunscreen active
ingredients apparently have not been studied. Although the available data are limited and not
conclusive, they give rise to questions about the safety and effectiveness of these products. Our
reasons for these tentative conclusions are detailed in the discussion that follows.
I. Public comments on the 2007 call for data
FDA received six submissions in response to the 2007 call for data. None of the
comments included substantive data, although some cited published scientific and medical
literature, addressed elsewhere in this document. Five of the six comments were from
manufacturers or a trade association. Industry comments generally favored retaining joint
regulation between EPA and FDA (perhaps with enhanced coordination and information-

Proposed Order OTC000008
Page 95
sharing) and amending the then-stayed OTC sunscreen monograph to address sunscreen-insect
repellent combinations. Several industry comments claimed there was an absence of conflicting
requirements relating to manufacturing, formulation, and/or labeling. Others suggested
approaches for minimizing labeling conflicts, such as permitting exemptions to FDA’s Drug
Facts labeling requirements to accommodate EPA-required information or placing FDA- and
EPA-required information in separate areas of the label. The remaining comment was submitted
by a medical association that opposed continued marketing of sunscreen-insect repellent
products, emphasizing concerns about children’s exposure to DEET. Industry comments
favoring the continued marketing of combination sunscreen-insect repellent drug products also
contended that combining sunscreen and insect repellent ingredients in a single product is more
convenient and cost-effective than using separate products. Two comments stated that properly
formulated, tested, and labeled, combination products are better than the unpredictable effects
that could arise when consumers use two different products. Regarding safety, one comment
asserted various flaws in the studies cited in the call for data that questioned the safety of these
combination products. (These studies are discussed in section VI.E.v.2.III.)
In general, the comments that we received in response to the 2007 call for data were not
accompanied or corroborated by data. Although the comments did not identify further concerns
relating to product manufacturing or formulation, they did not adequately address FDA’s
concerns about safety, effectiveness, and labeling of these products. FDA renews its request for
data to support labeling and safety for sunscreens with insect repellent added.
II. Disparities in required labeling of sunscreens
and insect repellents
FDA and EPA regulate the format and content of the labeling of nonprescription
sunscreen products and pesticides, respectively. FDA requirements governing nonprescription
sunscreen labeling include the general drug labeling regulations in subpart A of 21 CFR part
201; the “Drug Facts” format and other OTC drug labeling requirements in subpart C of 21 CFR
part 201; and the sunscreen-specific labeling requirements that apply to sunscreens marketed
without an approved NDA, including those based on the requirements for SPF and broad
spectrum testing in M020.
71
. The labeling of registered insect repellents is subject to EPA
labeling requirements under FIFRA (40 CFR 156), as well as specific language specified in
individual product registration documents. Although the FDA and EPA labeling requirements
for nonprescription sunscreens and registered pesticides cover some of the same information
(such as ingredient lists, net quantity statements, and warnings/precautions), there is considerable
variation in the language, format, and placement of common label elements between the two
Agencies, while other elements do not overlap.
71
The substance of relevant labeling provisions discussed below is unchanged between the Deemed Final Order and
this proposed order, although some provisions within § M020 are renumbered.
Proposed Order OTC000008
Page 96
Furthermore, both Agencies limit the degree to which a drug manufacturer or pesticide
registrant may depart from the prescribed text, format, and/or location of required labeling
elements. This is particularly true for the wording and format of “drug facts” information for
OTC drugs (see 21 CFR 201.66). Similarly, EPA regulations state that although a registrant may
choose to place non-FIFRA-required information on a pesticide label, it may not replace,
obscure, conflict with, or supersede the FIFRA-required text (EPA 2018).
The intended uses of sunscreens and insect repellents are quite different, as are the
associated labeling requirements; in particular, the instructions for using the two types of
products are different. Required labeling for OTC sunscreens marketed without approved NDAs
calls for reapplication at least every 2 hours (see § M020.50(e) of the Deemed Final Order). The
duration of protection for insect repellents varies according to the active ingredient and strength.
Based on information from the EPA product list, many insect repellent-sunscreen products
provide protection against mosquitoes and/or ticks for more than 2 hours, and some provide
protection for as many as 6 to 10 hours. The Centers for Disease Control and Prevention (CDC)
advises consumers that “products that combine sunscreen and repellent are not recommended,
because sunscreen may need to be reapplied more often and in larger amounts than needed for
the repellent component to provide protection from biting insects.” (CDC, n.d.). Similarly, the
American Academy of Pediatrics advises consumers not to use products that combine DEET
with sunscreen, in part because “[t]hese products can overexpose your child to DEET because
the sunscreen needs to be reapplied often” (American Academy of Pediatrics, n.d.).
Additionally, DEET is approved for use on children with no age restriction (EPA (n.d.),
“DEET”), whereas FDA labeling states “[bullet] children under 6 months of age, ask a doctor”
(see §M020.50(e) of the Deemed Final Order.
The recommended manner of application also differs for sunscreens and insect repellents.
For example, the directions on the label for all insect repellent products containing DEET say to
apply just enough to cover exposed skin, and avoid over-application (EPA (n.d.), “Using Insect
Repellents Safely and Effectively”), whereas the labeling of nonprescription sunscreens
marketed without approved NDAs calls for liberal or generous application (see § M020.50(e) of
the Deemed Final Order). The EPA-mandated directions on the labels of DEET products also
state, “Do not apply near eyes and mouth; apply sparingly around ears; do not use under
clothing” (EPA (n.d.), “Using Insect Repellents Safely and Effectively”). Such statements are
potentially troublesome from the standpoint of sun protection in light of surveillance data from
Australia, which suggest that the incidence of certain skin cancers is more frequent on highly
exposed areas of the body such as ears and the backs of hands (Kaldor et al. 1993; Girschik et al.
2008). The CDC advises consumers who need protection from both sun and insects to apply
sunscreen product first, followed by an insect repellent (CDC, n.d.).
Proposed Order OTC000008
Page 97
Additional disparities in the content and format of labeling elements for sunscreens and
registered insect repellents include the following:
• EPA pesticide labeling includes required elements that generally must appear on the
front panel of the label, such as the ingredient statement (40 CFR 156.10(g)(2)),
specified signal word such as “CAUTION” (40 CFR 156.64), and child hazard
warning (40 CFR 156.66), which could crowd or detract from drug information
required to appear on the principal display panel for drugs (see § 201.60 (“The
principal display panel shall be large enough to accommodate all the mandatory label
information required to be placed thereon by this part.”)). Other labeling elements
that only EPA requires include registration numbers and manufacturing establishment
numbers (40 CFR 156.10(a)).
• FDA labeling for sunscreens uses the word “warning” (see 21 CFR 201.66(c)(5) and
Deemed Final Order at § M020.50(d)), while the EPA requirements specify that
pesticide products that, like DEET, IR3535, and oil of citronella, meet the criteria of
Toxicity Category III or IV as the highest category by any route of exposure bear on
the front panel either no signal word or only the signal word “CAUTION” (40 CFR
156.64). In EPA labeling the word “WARNING” is used as a signal word only for
toxicity category II, which is a higher toxicity category than that applicable to any
insect repellent ingredients used in sunscreen-insect repellent combination products
(40 CFR 156.64(a)(2)).
• FDA labeling uses the term “directions” (see 21 CFR 201.66(c)(6) and Deemed Final
Order at § M020.50(e)), while EPA regulations use the term “directions for use” (see
40 CFR 156.10(i)(2)).
• FDA calls for ingredients to be listed as “active” and “inactive” (see 21 CFR
201.66(b) through (c)), while EPA labeling uses the term “inert” or “other” instead of
“inactive” for all non-pesticide ingredients (40 CFR 156.10(g)).
Given the extent of the disparities discussed above, FDA tentatively concludes that
attempting to merge the required labeling for monograph sunscreens and insect repellents in a
way that would comply with both Agencies’ requirements and permit adequate consumer
understanding and proper use would be impracticable. In this regard, we specifically disagree
with comments made in response to the 2007 FDA Call for Data suggesting that acceptable
“merged” labeling could be crafted by varying the OTC sunscreen drug facts to include insect-
repellent-related information, and/or by providing EPA-required labeling outside the drug facts
box. We are particularly concerned that consumers would be confused by the juxtaposition of
two sets of different and, in some cases, contradictory information in the labeling about these
products’ dual intended uses. We are also concerned that the sheer amount of required
information would result in crowded, difficult-to-read labels lacking in the clarity and
prominence of important safety and use information that are both required by FDA regulations
Proposed Order OTC000008
Page 98
and vital to consumer comprehension. We solicit comment and data about how to reconcile the
labeling of sunscreens and insect repellents such that a combined product could meet FD&C Act
requirements for OTC sunscreen drugs.
III. FDA’s review of published medical literature
The results of FDA’s literature review raise potential safety concerns about products that
combine sunscreen and insect repellent active ingredients. The available data suggest that the
dermal penetration and systemic absorption of at least one combination of a sunscreen active
ingredient and an insect repellent is increased when both are present.
There have been some studies assessing the penetration of DEET and the effects of
DEET combined with sunscreen (particularly the active ingredient oxybenzone) on dermal
penetration. Ross et al. tested for synergistic effects between DEET and oxybenzone using an in
vitro mouse skin diffusion model and showed substantial penetration of a 20 percent DEET
standard in ethanol, while penetration of sunscreen active ingredients was not found (Ross et al.
2004). Despite a lower DEET content (10 percent), a commercially marketed sunscreen
formulation had a 6-fold more rapid detection and a 3- to 4-fold greater penetration of DEET
than the 20 percent standard. Other diffusion tests using pigskin or artificial membranes and
various combinations of DEET and oxybenzone in different media suggested an enhancing effect
on dermal penetration of both DEET and oxybenzone (Gu et al. 2005; Wang and Gu 2007). The
same investigators obtained similar results in a later in vitro study using human skin (Wang and
Gu 2007).
Kasichayanula et al. assessed the dermal absorption of DEET and oxybenzone using an in
vivo piglet model, in which samples were collected from plasma, urine, and under the skin.
Their results indicated that the enhanced dermal penetration evidenced in the in vitro studies
translated to increased systemic exposure to both oxybenzone and DEET (Kasichayanula et al.
2005; Kasichayanula et al. 2007). Finally, a study by Yiin et al. (2015) suggests that enhanced
systemic absorption would also occur in humans. Yiin et al. used human urinary metabolites of
DEET and oxybenzone to evaluate the mutual enhancing effect on absorption of these
ingredients and concluded that their findings confirm that concurrent use of DEET-containing
insect repellent and oxybenzone-containing sunscreen results in the enhancement of dermal
absorption of DEET when insect repellent (DEET) was applied first and then covered by
sunscreen (Yiin et al. 2015). The study authors suggested that placing repellent spray on top of
sunscreen lotion with no mixing seems to be the best approach to diminish DEET penetration
through the skin.
Although insect repellents and sunscreens are designed to exert their protective effects on
the surface of the skin, the studies described above suggest that combining a sunscreen and insect
Proposed Order OTC000008
Page 99
repellent in a single product may result in unintended absorption of the sunscreen ingredient
oxybenzone and the insect repellent ingredient DEET, which in turn may impact effectiveness of
the product for use as sunscreen (discussed further below) as well as produce systemic exposure.
We acknowledge the study limitations cited by comments to the FDA Call for Data, and that in
vitro diffusion studies have their limitations in terms of reflecting clinical use. We also note that
many of the studies tested formulated commercial products with multiple sunscreen ingredients
and excipients for which details were not given, and it is unclear how this may have influenced
the results. Although we, therefore, do not view these data as conclusory, we have determined
that they raise a valid safety concern that warrants a tentative conclusion that, even if one could
overcome the misbranding and associated safety and effectiveness concerns created by the
inconsistent application directions for sunscreens and insect repellants, there would not be
sufficient evidence to conclude that combination sunscreen and insect repellent products are
GRASE for sunscreen use without further investigation (see FD&C Act section
505G(b)(1)(C)(ii)).
Regarding future investigations that could assist FDA in determining whether these
products have sufficient evidence of safety to be GRASE for use as sunscreen, we are not aware
of any data that define the extent of systemic exposure to oxybenzone that would occur with
maximal exposure to a sunscreen-insect repellent combination product. There also are few data
from which to assess whether there would be a similar enhancement of skin penetration for other
combinations of sunscreen and insect repellent active ingredients. Moreover, without adequate
human absorption studies under maximal use conditions of particular sunscreen-insect repellent
combinations (i.e., a MUsT, as discussed in section VI.C.iv), it is difficult to evaluate potential
risks associated with the use of such combination products. Because of the potential synergistic
interaction between the sunscreen active ingredient and the insect repellent active ingredient,
human absorption data for the individual components would not provide adequate data to
estimate the level of systemic absorption. Likewise, in vitro data would not be able to provide a
reliable estimate of the systemic exposure that would occur with such products’ use.
In terms of sunscreen active ingredient effectiveness, we have little data from which to
determine whether the presence of an insect repellent would affect the determined SPF value of
combination sunscreen-insect repellent products. Montemarano et al. reported a reduction in
sunscreen efficacy because of concomitant use with insect repellent. However, in that study, the
sunscreen and insect repellent ingredients were applied separately and were not part of a
combination product (Montemarano et al. 1997).
With respect to efficacy, we recognize that the efficacy testing required by the sunscreen
monograph, § M020 (both in the Deemed Final Order and if amended as proposed elsewhere in
this proposed order), could potentially mitigate concerns about the impact of insect repellent
active ingredients on sunscreen effectiveness. However, we are not aware of any data evaluating
Proposed Order OTC000008
Page 100
the reliability of SPF testing for sunscreen formulations that contain insect repellent ingredients.
There also is the possibility that increasing the amount of the sunscreen active ingredient to
compensate for any loss in efficacy because of the presence of the insect repellent could result in
unnecessarily high exposure to the sunscreen active ingredient. For these additional reasons, we
propose that even if other concerns could be overcome, these products are not GRASE because
there is not currently sufficient evidence to conclude that sunscreen-insect repellent products are
GRASE for use as sunscreens (see section 505G(b)(1)(C)(ii) of the FD&C Act). We solicit
comment on the data needs identified above and tentative conclusions, including supporting data
and analysis. We also solicit data and information to address these data needs.
IV. Conclusion
FDA tentatively concludes that the inherent disparity in labeling requirements that apply
to sunscreens marketed under the OTC monograph and insect repellents prevent the creation of
labeling that will sufficiently ensure safe and effective use of the sunscreen component of
sunscreen-insect repellent combination products, particularly in connection with duration of
action. For this reason, we propose that these products are not GRASE for use as sunscreens
under section 505G(b)(1)(C)(i) of the FD&C Act. We also note that these conflicting
requirements prevent these products from having adequate directions for use as a sunscreen, and
thus these products would be misbranded under section 502(f) of the FD&C Act. In addition,
even if these issues could be overcome, existing safety concerns about potential enhanced
systemic absorption resulting from combining individual sunscreen active ingredients and insect
repellent ingredients would also need to be addressed by further studies on both combinations of
individual sunscreen and insect repellent ingredients and final formulations. Additional data
would be needed to identify any interactions between specific sunscreen active ingredients and
insect repellents, in particular, to characterize any enhancement of skin penetration and/or
systemic absorption of the sunscreen component if the resulting data presents safety or
effectiveness concerns. As stated above, FDA would need adequate human absorption studies,
such as a MUsT, as part of the clinical safety assessment (for more discussion on assessment of
dermal absorption of sunscreen active ingredients using a MUsT program, see section VI.C.iv).
The effectiveness of sunscreen-insect repellent combination products is also a continuing
concern. For all of those reasons, we tentatively determine that these products are not GRASE
for nonprescription sunscreen use.
We solicit comment on this tentative determination.
vi. Other Proposals for Harmonization and/or Consolidation
1. Proposed Conditions for Products Containing Both Sunscreen
Active Ingredients and Skin Protectant Active Ingredients

Proposed Order OTC000008
Page 101
We are also proposing requirements for products containing both sunscreen active
ingredients and skin protectant active ingredients. These proposed requirements, if finalized,
would be included as provisions in the sunscreen monograph, with corresponding revisions to the
skin protectant monograph (in §§ M016.20, M016.50, and M016.60)).
First, we propose that the sunscreen monograph specify conditions that include
identifying which sunscreen active ingredient(s) (and in what concentrations) can be included in
a single product containing both skin protectant and sunscreen active ingredients (see proposed §
M020.20(b).) This is consistent with provisions already included in the skin protectant
monograph at § M016.20(e).
Second, we propose to include in both the sunscreen and skin protectant monographs
requirements for how such sunscreen-skin protectant products’ statements of identity,
indications, warnings, and directions are to be presented in labeling. (See proposed §§
M020.52(a), M020.60, M016.50(b) and M016.60.) These proposed requirements are consistent
with other labeling provisions in this proposed order.
2. Additional Proposals to Harmonize/Consolidate Provisions
We propose to consolidate under new § NM020 provisions describing certain properties
that render an OTC drug product offered for use as sunscreen a new drug for which an approved
NDA is required before marketing.
72
OTC Non-Monograph Conditions § NM900: Drug
Products Containing Certain Active Ingredients Offered Over-the-Counter for Certain Uses
73
currently contains several such provisions addressing specific ingredients and efficacy claims.
We propose to relocate the provisions pertaining to sunscreens from § NM900 to a new Non-
Monograph Conditions § NM020: Sunscreen Drug Products for Over-the-Counter Use.”
In addition, we are clarifying in § NM020 that labeling a product with claims that it
decreases the risk of skin cancer or early skin aging caused by the sun if that product has an SPF
of less than 15 when tested in accordance with § M020.80 and/or does not pass the broad
spectrum test in § M020.90 renders the product a new drug.
Finally, we propose to add to § NM020 new characteristics that FDA has determined
under section 505G(b)(1)(C) of the FD&C Act render sunscreens not GRASE. These
characteristics include: (1) containing the ingredients we propose to classify as not GRASE (see
72
Non-Monograph Conditions identify a non-exhaustive list of properties that would render the subject OTC drug
product a new drug. Therefore, the provisions in § NM020 should not be considered a comprehensive list of all
properties that render an OTC drug product offered for use as a sunscreen a new drug.
73
Non-Monograph Conditions § NM900, Drug Products Containing Certain Active Ingredients Offered Over-the-
Counter for Certain Uses, encompassed the provisions of 21 CFR 310.545 as in effect on March 26, 2020, which
were deemed to be a final administrative order by section 505G(k)(2)(A) of the FD&C Act upon its enactment.

Proposed Order OTC000008
Page 102
sections VI.C.ii-iii); (2) being labeled, represented, or promoted for use as a combined
sunscreen-insect repellant (see section VI.E.v); (3) failing to comply with provisions relating to
maximum SPF values and broad spectrum requirements (see section VI.E.ii); and (4) failing to
conform to certain other sunscreen formulation and dosage form conditions (see sections VI.E.i
and iv).
VII. PROPOSED EFFECTIVE DATE AND IMPLEMENTATION
APPROACH
Consistent with section 3854(c)(1)(B) of the CARES Act, Public Law 116-136, the
proposed effective date of final OTC monograph and OTC non-monograph provisions resulting
from the proposals described in this proposed order is 1 year after the date of publication of a
final order, subject to sections 505G(b)(2)(A)(iv)(I) and 505G(b)(3)(D)(ii) of the FD&C Act.
VIII. PROPOSED MONOGRAPH AND NON-MONOGRAPH
AMENDMENTS
Under section 505G(b) of the FD&C Act, and under the authority delegated to the
Commissioner of Food and Drugs, we propose to amend the OTC monographs § M016 (Skin
Protectant Drug Products for OTC Human Use) and § M020 (Sunscreen Drug Products for OTC
Human Use); and Non-Monograph Conditions § NM900 (Non-Monograph Conditions: Drug
Products Containing Certain Active Ingredients Offered Over-the-Counter for Certain Uses) and
to establish OTC Non-Monograph Conditions § NM020 (Non-Monograph Conditions § NM020:
Sunscreen Drug Products for Over-the-Counter Human Use).
We propose to amend OTC Monograph § M020, Sunscreen Drug Products for OTC
Human Use, by replacing the Deemed Final Order in its entirety with the following:
U.S. Food and Drug Administration
Over-the-Counter Monograph M020:
Sunscreen Drug Products for Over-the-Counter Human Use
(Proposal Issued September 24, 2021)
Part A—General Provisions
Sec.
M020.1 Scope
M020.3 Definitions
M020.5 Sun protection factor related conditions
Part B—Active Ingredients, Route of Administration, and Dosage Forms

Proposed Order OTC000008
Page 103
M020.10 Sunscreen active ingredients
M020.20 Permitted combinations of active ingredients
M020.30 Route of administration
M020.40 Dosage forms
Part C—Labeling
M020.50 Principal display panel of all sunscreen drug products
M020.52 Labeling of products containing one or more sunscreen active ingredients
M020.60 Labeling of products containing a combination of sunscreen and skin protectant active
ingredients
Part D—Final Formulation Testing
M020.70 General obligations of responsible persons
M020.80 Sun Protection Factor (SPF) testing
M020.90 Broad spectrum testing
M020.100 Regulatory status of final formulation testing and related requirements
M020.110 Recordkeeping
Part A—General Provisions
§ M020.1 Scope
An over-the-counter (OTC) sunscreen drug product is generally recognized as safe and effective
if it meets each condition in this OTC Monograph and each general condition established in 21
CFR 330.1.
§ M020.3 Definitions
As used in this OTC Monograph:
(a) Sunscreen active ingredient. An active ingredient that absorbs, reflects, or scatters radiation
in the ultraviolet (UV) range at wavelengths from 290 to 400 nanometers.
(b) Determined sun protection factor (SPF) value. The SPF value that equals the largest whole
number less than SPF
(
t SE
)
, determined for a sunscreen product in accordance with
§ M020.80.
(c) Labeled sun protection factor (SPF) value. The SPF value associated with the range into
which the determined SPF value falls, as set forth in the table in § M020.50(a)(2)(i).
(d) Extremely flammable. The term “extremely flammable” applies to any product that has a
flashpoint at or below 20
o
F (-6.7
o
C) as determined by the test method described at 16 CFR

Proposed Order OTC000008
Page 104
1500.43a, as published at 51 FR 28539 (August 8, 1986), except that any product having one
component or more with a flashpoint higher than 20
o
F (-6.7
o
C) that comprises at least 99
percent of the total volume of the product is not considered to be extremely flammable.
(e) Flammable. The term “flammable” applies to any product that has a flashpoint above 20
o
F (-
6.7
o
C) and below 100
o
F (37.8
o
C) as determined by the test method described at 16 CFR
1500.43a, as published at 51 FR 28539 (August 8, 1986), except that:
(1) Any product having one component or more with a flashpoint at or above 100
o
F
(37.8
o
C) that comprises at least 99 percent of the total volume of the product is not
considered to be flammable; and
(2) Any product containing 24 percent or less of water miscible alcohols, by volume, in
aqueous solution is not considered to be flammable if the product does not present a
significant flammability hazard when used by consumers.
(f) Combustible. The term “combustible” applies to any product having a flashpoint at or above
100
o
F (37.8
o
C) to and including 150
o
F (65.6
o
C) as determined by the test method described at
16 CFR 1500.43a, as published at 51 FR 28539 (August 8, 1986), except that:
(1) Any product having one component or more with a flashpoint higher than 150
o
F
(65.6
o
C) that comprises at least 99 percent of the total volume of the product is not
considered to be combustible; and
(2) Any product containing 24 percent or less of water miscible alcohols, by volume, in
aqueous solution is not considered to be combustible if the product does not present a
significant flammability hazard when used by consumers.
(g) Responsible person. A “responsible person” is the manufacturer, packer, or distributor whose
name appears on the labeling of a product covered by this OTC monograph.
§ M020.5 Sun protection factor related conditions
(a) The product has a determined SPF value of at least 2 but no greater than 80.
(b) If the product has a determined SPF value of at least 15, it also passes the broad spectrum test
in § M020.90.
Part B—Active Ingredients
§ M020.10 Sunscreen active ingredients
The active ingredient of the product consists of any of the following, under the conditions
specified, including being within the concentration specified for each ingredient:
(a) through (o) [Reserved]

Proposed Order OTC000008
Page 105
(p) Titanium dioxide up to 25 percent
(q) [Reserved]
(r) Zinc oxide up to 25 percent.
§ M020.20 Permitted combinations of active ingredients
(a) Combinations of sunscreen active ingredients. Two or more sunscreen active ingredients
identified in § M020.10 may be combined with each other in a single sunscreen product if all of
the following conditions are met:
(1) Each sunscreen active ingredient in the product must satisfy the conditions established
for its use in § M020.10.
(2) The concentration of each sunscreen active ingredient must be sufficient to contribute
a minimum determined SPF of not less than 2 to the finished product.
(3) The finished product must have a minimum determined SPF of not less than the
number of sunscreen active ingredients used in the product multiplied by 2.
(b) Combinations of sunscreen and skin protectant active ingredients. Any single sunscreen
active ingredient identified in § M020.10 or any combination of sunscreen active ingredients
permitted under § M020.20(a) may be combined with one or more skin protectant active
ingredients identified in §§ M016.10(a), (d), (e), (g), (h), (i), (k), (l), (m), and (r) of OTC
Monograph M016 when all of the following conditions are met:
(1) Each sunscreen active ingredient in the product must satisfy the conditions established
for its use in § M020.10.
(2) The concentration of each sunscreen active ingredient must be sufficient to contribute
a minimum determined SPF of not less than 2 to the finished product.
(3) The finished product must have a minimum determined SPF of not less than the
number of sunscreen active ingredients used in the product multiplied by 2.
(4) The product must be labeled according to §§ M020.50, M020.52, M020.60 and §
M016.60.
§ M020.30 Route of administration
The product is intended for topical administration.
§ M020.40 Dosage forms
Proposed Order OTC000008
Page 106
The product is in one of the following dosage forms and meets any additional conditions
specified:
(a) oil
(b) lotion
(c) cream
(d) gel
(e) butter
(f) paste
(g) ointment
(h) stick
(i) spray, provided that all of the following conditions are satisfied:
(1) Size of particles as dispensed from the consumer container:
(i) No more than 10 percent of the particles dispensed from the consumer
container are smaller than 10 micrometers; and
(ii) None of the particles dispensed from the consumer container are smaller than
5 micrometers.
(2) The product does not meet the definition of the term “extremely flammable” as
defined in § M020.3(d).
(3) The product is tested in accordance with 16 CFR 1500.43a as published at 51 FR
28539 (August 8, 1986), and if it meets the definition of either the term “flammable” or
the term “combustible” as defined at §§ M020.3(e) or (f), then the product also has a
measured drying time of less than 10 minutes.
(4) The product is labeled as required by §§ M020.52(b) and (c)(5).
(5) Testing in accordance with 21 CFR part 211 must confirm that the product meets the
conditions for particle size, flammability, and drying time as required by this OTC
Monograph and reflected in the product labeling.
(i) Testing of each lot of product for size of particles dispensed from the consumer
container must be conducted in accordance with adequate written specifications.

Proposed Order OTC000008
Page 107
(ii) Flammability testing for each batch of product must be conducted in
accordance with the specifications set forth in 16 CFR 1500.43a.
(iii) The product is tested accordance with 16 CFR 1500.43a, as published at 51
FR 28539 (August 8, 1986), and if it meets the definition of either the term
“flammable” or “combustible” as defined at §§ M020.3(e) or (f), as applicable,
drying time for each lot of product must be conducted in accordance with
adequate written specifications.
Part C—Labeling
§ M020.50 Principal display panel of all sunscreen drug products
(a) Principal display panel. The following labeling must be prominently placed on the principal
display panel:
(1) Statement of identity
(i) Placement. The principal display panel of an over-the-counter sunscreen drug
product bears a statement of identity as one of its principal features. Except as set
forth in § M020.60, the statement of identity consists of the established name(s)
of all sunscreen active ingredient(s) in the product, in alphabetical order and
followed by “Sunscreen” and “[Dosage form]” (e.g., “Lotion” “Spray”).
(ii) Prominence. The statement of identity must appear on the principal display
panel in boldface type at least one-quarter as large as the size of the most
prominent printed matter on the principal display panel, in lines generally parallel
to the base on which the package rests as it is designed to be displayed and in
direct conjunction with the most prominent display of the proprietary name or
designation. The entire text of the statement of identity must appear in the same
font style, size, and color with the same background color, and as continuous text
with no intervening text or graphic, other than additional text provided in
accordance with § M020.60.
(2) Effectiveness claim.
(i) SPF Broad Spectrum Statement. For a product that has been shown to pass the
broad spectrum test in § M020.90, the labeling states “Broad Spectrum SPF
[insert the labeled SPF value associated with the range into which the determined
SPF value falls, as set forth in the following table.]”
Table 1 to § M020.50(a)(2)(i)--SPF Labeling Ranges
Range of Determined SPF
Values
Associated Labeled SPF
Value
60-80
60+
50-59
50
Proposed Order OTC000008
Page 108
40-49
40
30-39
30
25-29
25
20-24
20
15-19
15
2-14
[Insert Determined SPF
Value]
(ii) SPF Statement. For a product that has not been shown to pass the broad
spectrum test in § M020.90, the labeling states “SPF [insert labeled SPF value
associated with the range into which the determined SPF value falls, as set forth
in the table in § M020.50(a)(2)(i)]”.
(iii) For a product with a determined SPF value of at least 2 but less than 15. The
SPF statement is immediately followed by an asterisk (“*”), and the associated
statement “*See Skin Cancer/Skin Aging Alert” appears in the bottom 30 percent
of the principal display panel.
(iv) Prominence of required statements. The SPF Broad Spectrum statement, SPF
statement, and “*See Skin Cancer/Skin Aging Alert” statement, as applicable,
must appear in boldface type at least one-quarter as large as the most prominent
printed matter on the principal display panel and in lines generally parallel to the
base on which the package rests as it is designed to be displayed. The entire text
of the Broad Spectrum SPF or SPF statement, as applicable, must appear in the
same font style, size, and color with the same background color and must appear
as continuous text with no intervening text or graphic. The entire text of the
“*See Skin Cancer/Skin Aging Alert” statement, as applicable, must appear in the
same font style, size, and color with the same background color and must appear
as continuous text with no intervening text or graphic.
(3) Water resistance statements—
(i) For products that provide 40 minutes of water resistance according to the test
in § M020.80(h)(1). The labeling states “Water Resistant (40 minutes).”
(ii) For products that provide 80 minutes of water resistance according to the test
in § M020.80(h)(2). The labeling states “Water Resistant (80 minutes).”
(iii) Prominence of water resistance statement. For all products bearing a water
resistance statement, the statement must appear in boldface type at least one-
quarter as large as the most prominent printed matter on the principal display
panel and in lines generally parallel to the base on which the package rests as it is
designed to be displayed. The entire text of the water resistance statement must
appear in the same font style, size, and color with the same background color, and
as continuous text with no intervening text or graphic.

Proposed Order OTC000008
Page 109
§ M020.52 Labeling of products containing one or more sunscreen active ingredients
(a) Indications. The labeling of the product states, under the heading “Uses,” the phrases listed in
§ M020.52(a), as appropriate. Other truthful and nonmisleading statements, describing only the
uses that have been established and listed in § M020.52(a) or uses permitted by § M020.60(b) for
products containing both sunscreen and skin protectant active ingredients, may also be used, as
provided in 21 CFR 330.1(c)(2).
(1) For all sunscreen products, the following indication statement must be included under
the heading “Uses”: “[bullet]
74
helps prevent sunburn”.
(2) For sunscreen products that have been shown to pass the broad spectrum test in §
M020.90 and have a determined SPF value of 15 or higher, the labeling may include the
following statement in addition to the indication in § M020.52(a)(1): “[bullet] if used as
directed with other sun protection measures (see Directions [in bold italic font]),
decreases the risk of skin cancer and early skin aging caused by the sun”.
(3) Labeling or promotional materials must not suggest or imply that the use, alone, of
any sunscreen reduces the risk of or prevents skin cancer or early skin aging.
(b) Warnings. The labeling of the product contains the following warnings under the heading
“Warnings”.
(1) For all sunscreen products.
(i) The labeling states “Do not use [bullet] on damaged or broken skin.”
(ii) The labeling states “When using this product [bullet] keep out of eyes. Rinse
with water to remove.”
(iii) The labeling states “Stop use and ask a doctor if [bullet] rash occurs.”
(2) For sunscreen products that are broad spectrum with determined SPF values of at least
2 but less than 15 according to the SPF test in § M020.80 or that have not been shown to
pass the broad spectrum test in § M020.90. The first statement under the heading
“Warnings” states “Skin Cancer/Skin Aging Alert [in bold font]: Spending time in the
sun increases your risk of skin cancer and early skin aging. This product has been shown
only to help prevent sunburn, not [in bold font] skin cancer or early skin aging.”
(3) For products in a spray dosage form that meet the definition of either the term
“flammable” or the term “combustible” as defined in §§ M020.3(e) or (f), as applicable,
74
See 21 CFR 201.66(b)(4) for definition of bullet

Proposed Order OTC000008
Page 110
when tested in accordance with 16 CFR 1500.43a, as published at 51 FR 28539 (August
8, 1986)
(i) Labeling statement. The labeling states [bullet] “Flammable” or
“Combustible” (as applicable) followed by a colon and the statement “Keep away
from fire or flame.”
(ii) For products that have a drying time of less than 5 minutes. The labeling
states [bullet] “Wait 5 minutes after application before approaching a source of
heat or flame, or before smoking.”
(iii) For products that have a drying time of at least 5 minutes but less than 10
minutes. The labeling states [bullet] “Wait 10 minutes after application before
approaching a source of heat or flame, or before smoking.”
(c) Directions. The labeling of the product contains the following statements, as appropriate,
under the heading “Directions.” More detailed directions applicable to a particular product
formulation may also be included.
(1) For all sunscreen products.
(i) As an option, the labeling may state “For sunscreen use:”.
(ii) The labeling states “[bullet] apply [select one of the following: ‘liberally’ or
‘generously’] [and, as an option: ‘and evenly’] 15 minutes before sun exposure”.
(iii) As an option, the labeling may state “[bullet] apply to all skin exposed to the
sun”.
(iv) The labeling states “[bullet] children under 6 months of age: Ask a doctor”.
(2) For sunscreen products that have been shown to pass the broad spectrum test in §
M020.90 and have a determined SPF value of 15 or higher. The labeling states “[bullet]
Sun Protection Measures. [in bold font] Spending time in the sun increases your risk of
skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a
Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
[bullet] limit time in the sun, especially from 10 a.m.-2 p.m. [bullet] wear long-sleeved
shirts, pants, hats, and sunglasses”.
(3) For products that satisfy the water resistance test in § M020.80(h). The labeling states
“[bullet] reapply: [bullet] after [select one of the following determined by water
resistance test: ‘40 minutes of’ or ‘80 minutes of’] swimming or sweating [bullet]
immediately after towel drying [bullet] at least every 2 hours”.

Proposed Order OTC000008
Page 111
(4) For products that do not satisfy the water resistance test in § M020.80(h). The
labeling states “[bullet] reapply at least every 2 hours [bullet] use a water resistant
sunscreen if swimming or sweating”.
5) For sunscreen products in a spray dosage form. The labeling states “[bullet] Hold
container 4 to 6 inches from the skin to apply. [bullet] Do not spray directly into face.
Spray on hands then apply to face. [bullet] Do not apply in windy conditions. [bullet]
Use in a well-ventilated area and avoid inhalation”.
(d) Other Information. The labeling of the product contains the following statement under the
heading “Other information:” “[bullet] protect the product in this container from excessive heat
and direct sun”.
(e) False or misleading claims. The labeling of the product must not contain claims that the
product is “sunblock,” “sweatproof,” “waterproof” or similar claims, which are false and/or
misleading with respect to sunscreens.
§ M020.60 Labeling of products containing a combination of sunscreen and skin protectant
active ingredients
Statements of identity, indications, warnings, and directions for use, respectively, applicable to
each ingredient in the product may be combined to eliminate duplicative words or phrases so that
the resulting information is clear and understandable. Labeling provisions in § M016.50(e) of
OTC Monograph M016 do not apply to these products.
(a) Statement of identity. The statement of identity of a sunscreen product that also contains one
or more skin protectant active ingredients, identified in §§ M016.10(a), (d), (e), (g), (h), (i), (k),
(l), (m), and (r) of OTC Monograph M016, consists of the established names of all sunscreen and
skin protectant active ingredients in alphabetical order followed by “Sunscreen/Skin Protectant”
and “[Dosage form].” The statement of identity must be prominently placed on the principal
display panel and presented in accordance with § M020.50(a)(1).
(b) Indications. The labeling of the product states, under the heading “Uses,” any or all of the
applicable indication(s) included in § M016.50(b)(2) of OTC Monograph M016 and the
indication required by § M020.52(a)(1). Any other indication in § M020.52(a) may also be
included if the product satisfies requirements for bearing that indication under that section. Other
truthful and nonmisleading statements, describing only the indications for use applicable to the
product that have been established in § M016.50(b)(2) of OTC Monograph M016 or in §
M020.52(a), may also be used, consistent with 21 CFR 330.1(c)(2).
(c) Warnings. The labeling of the product states, under the heading “Warnings,” the applicable
warnings for sunscreens in § M020.52(b) and for skin protectants in § M016.50(c) of OTC
Monograph M016.
(d) Directions. The labeling of the product states, under the heading “Directions,” any or all of
the applicable directions for sunscreens, as set forth in § M020.52(c), and for skin protectants, as

Proposed Order OTC000008
Page 112
set forth in § M016.50(d)) of OTC Monograph M016, unless otherwise stated in § M020.60(d).
When the time intervals or age limitations for administration of the individual ingredients differ,
the directions for the product may not contain any dosage that exceeds those established for any
individual ingredient in the applicable OTC monograph(s), and may not provide for use by any
age group lower than the highest minimum age limit established for any individual ingredient.
When the directions for administration of the sunscreen and skin protectant differ in any other
way, the directions for sunscreens in § M020.52(c) should be used.
Part D—Final Formulation Testing
§ M020.70 General obligations of responsible persons
A responsible person must assure that final formulation testing conducted on its product(s)
pursuant to §§ M020.80 and M020.90 complies with all applicable provisions of this OTC
Monograph.
(a) Transfer of obligations
(1) A responsible person may transfer responsibility for any or all of its obligations set
forth in this OTC monograph to another entity (e.g., a contract research organization
and/or testing laboratory), except as set forth in § M020.110 (recordkeeping). Any such
transfer must be described in writing. If not all obligations are transferred, the writing is
required to describe each of the obligations being assumed by the transferee. If all
obligations are transferred, a general statement that all obligations have been transferred
is acceptable. Any obligation not covered by the written description will be deemed not
to have been transferred. A written record of the transfer of obligations must be
maintained by both parties to the transfer for the time period set forth in § M020.110.
(2) An entity that assumes any obligation(s) of a responsible person must comply with the
provisions of this OTC monograph applicable to the assumed obligation and will be
subject to the same regulatory action as a responsible person for failure to comply with
any obligation assumed under this OTC Monograph. Thus, all references to “responsible
person” in this OTC Monograph apply to another entity (e.g., a contract research
organization or testing laboratory) to the extent that it assumes one or more obligations of
a responsible person.
(b) Personnel. A responsible person must select only investigators and other personnel qualified
by appropriate training and/or experience to conduct final formulation testing pursuant to this
OTC Monograph. Personnel engaged in testing under this OTC Monograph must have the
education, training, and experience, or any combination thereof, to enable that person to
adequately perform their assigned functions.
§ M020.80 Sun Protection Factor (SPF) testing
(a) Adequate clinical testing procedures and conditions—

Proposed Order OTC000008
Page 113
(1) General obligations of responsible persons for testing under § M020.80. Responsible
persons must provide investigators and other personnel engaged in SPF testing with the
information they need to conduct an investigation properly; must obtain a signed
investigator statement from each investigator; must ensure proper monitoring of the
investigation(s); must ensure that the investigation(s) is conducted in accordance with
written general investigational plan(s) and protocol(s); must ensure compliance with §§
M020.80(a)(2) and (a)(3); and must ensure that the Food and Drug Administration (FDA)
and all participating investigators are promptly informed of significant new adverse
effects or risks with respect to the drug.
(2) Informed consent. Legally effective informed consent, as defined in 21 CFR part 50,
must be obtained from all human subjects before initiating clinical final formulation
testing under § M020.80.
(3) Institutional Review Board (IRB) approval. Clinical testing under § M020.80, must
be reviewed and approved by an IRB meeting the requirements of FDA’s regulations in
21 CFR part 56.
(4) Control of personnel.
(i) General obligations. A responsible person is responsible for ensuring that
investigators and other personnel conducting any testing under § M020.80,
conduct all investigations in accordance with the signed investigator statement,
the investigational plan, and applicable regulations. Responsible persons must
ensure the implementation of adequate safeguards to protect the rights, safety, and
welfare of subjects under the investigator’s care. The responsible person must
also ensure that investigators or other study personnel will promptly report to the
IRB all changes in the clinical final formulation testing and all unanticipated
problems involving risk to human subjects or others, and that investigators or
other personnel will not make any changes in the clinical final formulation testing
without IRB approval, except where necessary to eliminate apparent immediate
hazards to human subjects.
(ii) Obtaining information from the investigator. Before permitting an
investigator to begin participating in clinical final formulation testing under §
M020.80, the responsible person must obtain the following:
(A) Investigator statement. A signed investigator statement containing the
name and address of the investigator and a commitment by the
investigator that he or she –
(1) Will conduct the testing in accordance with the relevant,
current protocol(s) and will only make changes in a protocol after
notifying the responsible person and the IRB, except when
necessary to protect the safety, the rights, or welfare of subjects;

Proposed Order OTC000008
Page 114
(2) Will comply with all requirements regarding the obligations of
clinical investigators and all other pertinent requirements in §§
M020.80 and M020.110;
(3) Will personally conduct or supervise the described
investigation(s);
(4) Will inform any potential subjects that the drugs are being used
for investigational purposes and will comply with the requirements
relating to obtaining informed consent (21 CFR part 50) and IRB
review and approval (21 CFR part 56);
(5) Will report to the responsible person adverse experiences that
occur during the investigation(s);
(6) Will ensure that all personnel assisting in the conduct of the
testing are informed about their obligations in meeting the above
commitments.
(B) Curriculum vitae. A curriculum vitae or other statement of
qualifications of the investigator showing the education, training, and
experience that qualifies the investigator to conduct the final formulation
testing pursuant to § M020.80.
(5) Informing investigators. The responsible person must, as the overall investigation
proceeds, keep each participating investigator informed of new observations discovered
by or reported to the responsible person on the drug, particularly with respect to adverse
effects and safe use.
(6) Review of ongoing investigations.
(i) The responsible person must monitor the progress of all clinical testing being
conducted on its final formulation pursuant to § M020.80.
(ii) A responsible person who discovers noncompliance by an investigator or
other personnel with the signed agreement, the general investigational plan, or the
requirements of § M020.80 or applicable regulations (e.g., 21 CFR parts 50 and
56) must promptly either secure compliance or end the investigator’s or other
personnel’s participation in testing conducted under § M020.80.
(iii) The responsible person must review and evaluate the evidence relating to the
safety and effectiveness of the final formulation as it is obtained from the
investigator.
(7) Investigator reports.
Proposed Order OTC000008
Page 115
(i) Safety reports. An investigator must immediately report to the responsible
person any serious adverse event, whether or not considered related to the final
formulation, including those listed in the protocol, and must include an
assessment of whether there is a reasonable possibility that the final formulation
being tested caused the adverse event. The investigator must record nonserious
adverse events and report them to the responsible person according to the
timetable specified in the protocol.
(ii) Final report. An investigator must provide the responsible person with an
adequate report shortly after completion of each investigation conducted by that
investigator for the responsible person under § M020.80.
(b) UV source (solar simulator).
(1) Emission spectrum. Filter a single port or multiport solar simulator so that it provides
a continuous emission spectrum from 290 to 400 nanometers (nm) with a limit of 1,500
watts per square meter (W/m
2
) on total irradiance for all wavelengths between 250 and
1,400 nm.
(i) The solar simulator must have the following percentage of erythema-effective
radiation in each specified range of wavelengths:
Table 2 to § M020.80(b)(1)(i)--Solar Simulator Emission Spectrum
Wavelength range (nm)
Percent erythemal contribution
1
<290
<0.1
290-300
1.0-8.0
290-310
49.0-65.0
290-320
85.0-90.0
290-330
91.5-95.5
290-340
94.0-97.0
290-400
99.9-100.0
1
Calculation of erythema action spectrum described in § M020.80(b)(2).
(ii) In addition, UVA II (320-340 nm) irradiance must equal or exceed 20 percent
of the total UV (290-400 nm) irradiance. UVA I (340-400 nm) irradiance must
equal or exceed 60 percent of the total UV irradiance.
(2) Erythema action spectrum.
(i) Calculate the erythema action spectrum weighting factor (V
i
) at each
wavelength λ:

Proposed Order OTC000008
Page 116
(A) V
i
(λ) = 1.0 (250 <λ ≤298 nm)
(B) V
i
(λ) = 10
0.094
* (
298
−λ) (298 <λ ≤328 nm)
(C) V
i
(λ) = 10
0.015
* (
140
−λ) (328 <λ ≤400 nm)
(ii) Calculate the erythema-effective UV dose (E) delivered by a solar simulator
as follows:
E = V
(
)
I
(
)
t
Where
V
i
(λ) = erythema action spectrum weighting factor at each wavelength λ
I(λ) = irradiance (Watts per square meter) at each wavelength λ
t = exposure time (seconds)
Erythema-effective dose (E) is expressed as effective Joules per square
meter (J/m
2
-eff).
(iii) The solar simulator radiation intensity must be determined using a handheld
radiometer with a response weighted to match the spectrum in ISO/CIE
17166:2019(E) entitled "Erythema reference action spectrum and standard
erythema dose," dated 2019 (First edition, 2019-05)), which is incorporated by
reference and is available for inspection at FDA. For further information about
inspecting incorporated materials, see the OTC Monographs@FDA portal at
https://www.accessdata.fda.gov/scripts/cder/omuf/index.cfm. Copies may also be
available from the publisher. The solar simulator output should be measured
before and after each phototest or, at a minimum, at the beginning and end of each
test day. This radiometer should be calibrated using side-by-side comparison with
the spectroradiometer (using the weighting factors determined according to §
M020.80(b)(2)(i)) at the time of the annual spectroradiometric measurement of
the solar simulator as described in § M020.80(b)(4).
(3) Operation. A solar simulator must have no significant time-related fluctuations
(within 20 percent) in radiation emissions after an appropriate warm-up time and
demonstrate good beam uniformity (within 20 percent) in the exposure plane. The
delivered dose to the UV exposure site must be within 10 percent of the expected dose.
(4) Periodic measurement. To ensure that the solar simulator delivers the appropriate
spectrum of UV radiation, the emission spectrum of the solar simulator must be measured
at least annually with an appropriate and accurately calibrated spectroradiometer system
(results should be traceable to the National Institute for Standards and Technology). In
addition, the solar simulator must be recalibrated if there is any change in the lamp bulb
Proposed Order OTC000008
Page 117
or the optical filtering components (i.e., filters, mirrors, lenses, collimating devices, or
focusing devices). Daily solar simulator radiation intensity should be monitored with a
broadband radiometer with a response weighted to match the erythema action spectrum in
ISO/CIE 17166:2019(E) entitled "Erythema reference action spectrum and standard
erythema dose,"” which is incorporated by reference in § M020.80(b)(2)(iii). If a lamp
must be replaced due to failure or aging during a phototest, broadband device readings
consistent with those obtained for the original calibrated lamp will suffice until
measurements can be performed with the spectroradiometer at the earliest possible
opportunity.
(c) SPF standard.
(1) Preparation. The SPF standard must be a formulation containing 7-percent padimate
O and 3-percent oxybenzone.
Table 3 to § M020.80(c)(1)--Composition of the Padimate O/Oxybenzone SPF Standard
Ingredients
Percent by weight
Part A:
Lanolin
4.50
Cocoa butter
2.00
Glyceryl monostearate
3.00
Stearic acid
2.00
Padimate O
7.00
Oxybenzone
3.00
Part B:
Purified water USP
71.60
Sorbitol solution
5.00
Triethanolamine, 99 percent
1.00
Methylparaben
0.30
Propylparaben
0.10
Part C:
Benzyl alcohol
0.50
Part D:
Purified water USP
QS
1
1
Quantity sufficient to make 100 grams.
Proposed Order OTC000008
Page 118
(i) Step 1. Add the ingredients of Part A into a suitable stainless steel kettle
equipped with a propeller agitator. Mix at 77 to 82 °C until uniform.
(ii) Step 2. Add the water of Part B into a suitable stainless steel kettle equipped
with a propeller agitator and begin mixing at 77 to 82 °C. Add the remaining
ingredients of Part B and mix until uniform.
(iii) Step 3. Add the batch of Step 1 to the batch of Step 2 and mix at 77 to 82 °C
until smooth and uniform. Slowly cool the batch to 49 to 54 °C.
(iv) Step 4. Add the benzyl alcohol of Part C to the batch of Step 3 at 49 to 54 °C.
Mix until uniform. Continue to cool batch to 35 to 41 °C.
(v) Step 5. Add sufficient water of Part D to the batch of Step 4 at 35 to 41 °C to
obtain 100 grams of SPF standard. Mix until uniform. Cool batch to 27 to 32 °C.
(2) HPLC assay. Use the following high performance liquid chromatography (HPLC)
procedure to verify the concentrations of padimate O and oxybenzone in the SPF
standard:
(i) Instrumentation.
(A) Equilibrate a suitable liquid chromatograph to the following or
equivalent conditions:
(1) Column
C-18, 250 millimeters (mm) length, 4.6 mm inner diameter (5 microns)
(2) Mobile Phase
85:15:0.5 methanol: water: acetic acid
(3) Flow Rate
1.5 milliliters (mL) per minute
(4) Temperature
Ambient
(5) Detector
UV spectrophotometer at 308 nanometers
(6) Attenuation
As needed
(B) Use HPLC grade reagents for mobile phase.
(ii) Preparation of the HPLC reference standard.
(A) Weigh 0.5 gram (g) of oxybenzone USP reference standard into a 250-
mL volumetric flask. Dissolve and dilute to volume with isopropanol.
Mix well.
(B) Weigh 0.5 g of padimate O USP reference standard into a 250-mL
volumetric flask. Dissolve and dilute to volume with isopropanol. Mix
well.
Proposed Order OTC000008
Page 119
(C) Pipet 3 mL of the oxybenzone solution and 7 mL of the padimate O
solution into a 100-mL volumetric flask. Dilute to volume with
isopropanol and mix well.
(iii) HPLC system suitability.
(A) Make three replicate 10-microliter injections of the HPLC reference
standard (described in § M020.80(c)(2)(ii)). The relative standard
deviation in peak areas should not be more than 2 percent for either
oxybenzone or padimate O.
(B) Calculate the resolution (R) between the oxybenzone and padimate O
peaks from one chromatogram as follows:
R =
2 (t
t
)
W
+ W
Where
t
o
= retention time for oxybenzone
t
p
= retention time for padimate O
W
o
= oxybenzone peak width at baseline
W
p
= padimate O peak width at baseline
If the resolution (R) is less than 3, adjust the mobile phase or
replace the column.
(iv) SPF standard assay.
(A) The SPF standard is diluted to the same concentration as the HPLC
reference standard according to the following steps:
(1) Step 1. Weigh 1 g of the SPF standard (described in §
M020.80(c)(1)) into a 50-mL volumetric flask.
(2) Step 2. Add approximately 30 mL of isopropanol and heat
with swirling until contents are evenly dispersed.
(3) Step 3. Cool to room temperature (15 to 30 °C) and dilute to
volume with isopropanol. Mix well.
(4) Step 4. Pipet 5.0 mL of the preparation into a 50-mL
volumetric flask and dilute to volume with isopropanol. Mix well.

Proposed Order OTC000008
Page 120
(B)(1) Inject 10-microliter of diluted SPF standard from §
M020.80(c)(2)(iv)(A) and calculate the amount of oxybenzone and
padimate O as follows:
Percent Oxybenzone =
Peak area of oxybenzone in sunscreen standard
Peak area of oxybenzone in HPLC reference standard
100
Percent Padimate O =
Peak area of padimate O in sunscreen standard
Peak area of padimate O in HPLC reference standard
100
(2) The percent of oxybenzone and padimate O in the SPF standard
must be between 95 and 105.
(d) Test subjects.
(1) Number of subjects. A test panel should include enough subjects to produce a
minimum of 10 valid test results. A maximum of three subjects may be rejected from
this panel based on § M020.80(f)(5).
(2) Medical history.
(i) Obtain a medical history from each subject with emphasis on the effects of
sunlight on the subject’s skin. Determine that each subject is in good general
health with skin type I, II, or III as follows; if these conditions are not met, the
subject is not qualified to participate in the study:
(A) Always burns easily; never tans (Skin type I: sensitive).
(B) Always burns easily; tans minimally (Skin type II: sensitive).
(C) Burns moderately; tans gradually (light brown) (Skin type III:
normal).
(D) Burns minimally; always tans well (moderate brown) (Skin type IV:
normal).
(E) Rarely burns; tans profusely (dark brown) (Skin type V: insensitive).
(F) Never burns; deeply pigmented (Skin type VI: insensitive).
(ii) Skin type is based on first 30 to 45 minutes of sun exposure after a winter
season of no sun exposure. Determine that each subject is not taking topical or
systemic medication that is known to alter responses to UV radiation. Determine

Proposed Order OTC000008
Page 121
that each subject has no history of sensitivities to topical products and/or
abnormal responses to sunlight, such as a phototoxic or photoallergic response.
(3) Physical examination. Conduct a physical examination to determine the presence of
sunburn, suntan, scars, active dermal lesions, and uneven skin tones on the areas of the
back to be tested. Adequate time must have passed following any previous UV exposure
(e.g., participation in a prior SPF clinical study, tanning, etc.) so that the test subject has
no preexisting skin pigmentation at the time of enrollment. A suitable source of low
power UVA, such as a Woods lamp, is helpful in this process. If any of these conditions
are present, the subject is not qualified to participate in the study. The presence of nevi,
blemishes, or moles will be acceptable if, in the physician’s judgment, they will neither
compromise the study nor jeopardize a subject’s safety. Subjects with dysplastic nevi
should not be enrolled. Excess hair on the back is acceptable if the hair is clipped.
Shaving is unacceptable because it may remove a significant portion of the stratum
corneum and temporarily alter the skin’s response to UV radiation.
(4) Informed consent. Obtain legally effective written informed consent from all test
subjects as required by § M020.80(a)(2).
(e) Sunscreen application.
(1) Test site. Test sites are locations on each subject’s back, between the beltline and the
shoulder blades (scapulae) and lateral to the midline, where skin responses to UV
radiation are determined. Responses on unprotected skin (no test material applied) and
protected skin (sunscreen test product(s) or SPF standard applied) are determined at
separate unprotected and protected test sites, respectively. Test sites should be randomly
located in a blinded manner. Each test site should be a minimum of 30 square
centimeters and outlined with indelible ink.
(2) Test subsite. Test subsites are the locations to which UV radiation is administered
within a test site. Administer UV doses to at least five test subsites within each test site.
Test subsites must be at least 0.5 square centimeters (cm
2
) in area and must be separated
from each other by at least 0.8 cm. Each test subsite must be outlined with indelible ink.
(3) Applying test materials. Apply the sunscreen test product and the SPF standard at 2
milligrams per square centimeter (mg/cm
2
) to their respective test sites. Use a finger cot
compatible with the sunscreen to spread the product as evenly as possible.
(4) Waiting period. Wait at least 15 minutes after applying a sunscreen product before
exposing the test sites to UV radiation as described in § M020.80(f). For water resistant
sunscreen products, proceed with the water resistance testing procedure described in §
M020.80(h) after waiting at least 15 minutes.
(f) UV exposure and erythema reading.

Proposed Order OTC000008
Page 122
(1) Definition of minimal erythema dose (MED). The following terms are defined for
purposes of OTC monograph M020. The minimal erythema dose (MED) is the smallest
UV dose (quantity of erythema-effective energy expressed as Joules per square meter)
that produces perceptible redness of the skin (erythema) with clearly defined borders at
16 to 24 hours after UV exposure. The MED for unprotected skin (MED
u
) is determined
on a test site that does not have sunscreen applied. The MED for protected skin (MED
p
)
is determined on a test site that has sunscreen applied. An MED
p
is determined for the
SPF standard (ssMED
p
). An MED
p
is determined for the sunscreen test product
(tpMED
p
).
(2) UV exposure for initial MED
u
. For each test subject, no more than 1 day before
testing a product, determine the initial MED
u
by administering a series of UV radiation
doses expressed as J/m
2
-eff (as determined according to § M020.80(b)(2)(ii)) to the test
subsites within an unprotected test site using an accurately calibrated solar simulator.
Select doses that are a geometric series represented by 1.25
n
(i.e., each dose is 25 percent
greater than the previous dose).
(3) UV exposure for final MED
u
,
ss
MED
p
, and
tp
MED
p
. For each subject, determine the
final MED
u
,
ss
MED
p
, and
tp
MED
p
by administering a series of five UV doses to the
appropriate test sites. The middle dose (X) in each of these dose series (i.e., the third
dose) should equal the initial MED
u
times the expected SPF. Note that the expected SPF
equals 1 and 16.3 for the final MED
u
and ssMED
p
, respectively. The remaining UV
doses in the series depend upon the expected SPF value of the sunscreen test product(s).
For products with an expected SPF less than 8, administer UV doses that increase by 25
percent with each successive dose (i.e., 0.64X, 0.80X, 1.00X, 1.25X, and 1.56X). For
products with an expected SPF from 8 to 15, administer UV doses that increase by 20
percent with each successive dose (i.e., 0.69X, 0.83X, 1.00X, 1.20X, and 1.44X). For
products with an expected SPF higher than 15, administer UV doses that increase by 15
percent with each successive dose (i.e., 0.76X, 0.87X, 1.00X, 1.15X, and 1.32X).
(4) Evaluation of test subsites. In order that the study personnel who evaluates the test
subsites is not biased, he/she should not be the same study personnel who applied the
sunscreen product to the test site or administered the UV doses. After UV doses are
administered, record all immediate responses. These may include an immediate
darkening or tanning, typically grayish or purplish in color, which fades in 30 to 60
minutes; an immediate reddening at the subsite, due to heating of the skin, which fades
rapidly; and an immediate generalized heat response, spreading beyond the subsite,
which fades in 30 to 60 minutes. After the immediate responses are noted, each subject
should shield the exposed area from further UV radiation until the MED is determined.
Determine the final MED
u
,
ss
MED
p
, and
tp
MED
p
16 to 24 hours after UV exposure.
Evaluate the erythema responses of each test subsite using either tungsten or warm white
fluorescent lighting that provides at least 450 lux of illumination at the test site. For the
evaluation, the test subject should be in the same position as when the test site was
irradiated.

Proposed Order OTC000008
Page 123
(5) Invalid test data. Reject test data for a test subject if erythema is not present on either
the unprotected or protected test sites; or erythema is present at all subsites; or the
responses are inconsistent with the series of UV doses administered; or the subject was
noncompliant (e.g., the subject withdraws from the test due to illness or work conflicts or
does not shield the exposed testing sites from further UV radiation until the MED is
determined).
(g) Determination of SPF.
(1) Calculate an SPF value for each test subject (SPF
i
) as follows:
SPFi =
MEDp
final MEDu
(2) Calculate the mean
SPF (SPF
)
and the standard deviation(s) from the SPF
i
values. Calculate the standard error (SE),
which equals s/√n (where n equals the number of subjects who provided valid test
results). Obtain the t value from Student’s t distribution table corresponding to the upper
5-percent point with n-1 degrees of freedom. Determine the SPF value that is equal to
the largest whole number less than
SPF
(t SE).
In order for the SPF determination of a test product to be considered valid, the SPF value
of the SPF standard must fall within the standard deviation range of the expected SPF
(i.e., 16.3 ±3.43).
(h) Determination of water resistance. To support labeling claims of water resistance in
accordance with § M020.50(a), the following procedure must be performed in an indoor fresh
water pool, whirlpool, and/or hot tub maintained at 23 to 32 °C. Fresh water is clean drinking
water that meets the standards in 40 CFR part 141. The pool and air temperature and the relative
humidity must be recorded.
(1) Water resistance (40 minutes). Determine the SPF value after 40 minutes of water
immersion using the following procedure:
(i) Step 1: Apply the sunscreen test product as described in § M020.80(e).
(ii) Step 2: Perform moderate activity in water for 20 minutes.
(iii) Step 3: Rest out of water for 15 minutes. Do not towel test site(s).

Proposed Order OTC000008
Page 124
(iv) Step 4: Perform moderate activity in water for 20 minutes.
(v) Step 5: Allow test sites to dry completely without toweling.
(vi) Step 6: Apply the SPF standard as described in § M020.80(e).
(vii) Step 7: Expose test sites to UV doses as described in § M020.80(f).
(2) Water resistance (80 minutes). Determine the SPF value after 80 minutes of water
immersion using the following procedure:
(i) Step 1: Apply the sunscreen test product as described in § M020.80(e).
(ii) Step 2: Perform moderate activity in water for 20 minutes.
(iii) Step 3: Rest out of water for 15 minutes. Do not towel test site(s).
(iv) Step 4: Perform moderate activity in water for 20 minutes.
(v) Step 5: Rest out of water for 15 minutes. Do not towel test site(s).
(vi) Step 6: Perform moderate activity in water for 20 minutes.
(vii) Step 7: Rest out of water for 15 minutes. Do not towel test site(s).
(viii) Step 8: Perform moderate activity in water for 20 minutes.
(ix) Step 9: Allow test sites to dry completely without toweling.
(x) Step 10: Apply the SPF standard as described in § M020.80(e).
(xi) Step 11: Expose test sites to UV doses as described in § M020.80(f).
§ M020.90 Broad spectrum testing
(a) UV Spectrometry
(1) Plate. Use optical-grade polymethylmethacrylate (PMMA) plates suitable for UV
transmittance measurements. The plate should be roughened on one side to a three-
dimensional surface topography measure (Sa) between 2 and 7 micrometers and must
have a rectangular application area of at least 16 square centimeters (with no side shorter
than 4 cm).
(2) Sample holder. The sample holder should hold the PMMA plate in a horizontal
position to avoid flowing of the sunscreen product from one edge of the PMMA plate to

Proposed Order OTC000008
Page 125
the other. Mount the PMMA plate as close as possible to the input optics of the
spectrometer to maximize capture of forward scattered radiation. The sample holder
should be a thin, flat plate with a suitable aperture through which UV radiation can pass.
Place the PMMA plate on the upper surface of the sample holder with the roughened side
facing up.
(3) Light source. The light source must produce a continuous spectral distribution of UV
radiation from 290 to 400 nanometers.
(4) Input optics. Unless the spectrometer is equipped with an integrating sphere, an
ultraviolet radiation diffuser should be placed between the sample and the input optics of
the spectrometer. The diffuser will be constructed from any UV radiation transparent
material (e.g., Teflon or quartz). The diffuser ensures that the radiation received by the
spectrometer is not collimated. Set the spectrometer input slits to provide a bandwidth
that is less than or equal to 5 nanometers.
(5) Dynamic range of the spectrometer. The dynamic range of the spectrometer should
be sufficient to measure transmittance accurately through a highly absorbing sunscreen
product at all terrestrial solar UV wavelengths (290 to 400 nm).
(b) Sunscreen product application to PMMA plate. The accuracy of the test depends upon the
application of a precisely controlled amount of sunscreen product with a uniform distribution
over the PMMA plate. The product is applied at 0.75 mg per square centimeter to the roughened
side of the PMMA plate. The sunscreen product should be applied in a series of small amounts
over the entire PMMA plate and then spread evenly using a gloved finger. Spreading should be
done with a very light spreading action for approximately 30 seconds followed by spreading with
greater pressure for approximately 30 seconds. The plate should then be allowed to equilibrate
for 15 minutes in the dark before the pre-irradiation described in § M020.90(c).
(c) Sunscreen product pre-irradiation. To account for lack of photostability, irradiate the PMMA
plate with a solar simulator described in § M020.80(b). The irradiation dose must be 4 MEDs
which is equivalent to an erythemal effective dose of 800 J/m
2
(i.e.,800 J/m
2
-eff).
(d) Calculation of mean transmittance values.
(1) After pre-irradiation, determine the mean transmittance values for each wavelength λ
over the full UV spectrum (290 to 400 nanometers). Measure the transmittance values at
1 nanometer intervals. Measurements of spectral irradiance transmitted for each
wavelength λ through control PMMA plates coated with 15 microliters of glycerin (no
sunscreen product) must be obtained from at least five different locations on the PMMA
plate [C1(λ), C2(λ), C3(λ), C4(λ), and C5(λ)]. In addition, a minimum of five
measurements of spectral irradiance transmitted for each wavelength λ through the
PMMA plate covered with the sunscreen product will be similarly obtained after pre-
irradiation of the sunscreen product [P1(λ), P2(λ), P3(λ), P4(λ), and P5(λ)].

Proposed Order OTC000008
Page 126
(2) The mean transmittance for each wavelength is the ratio of the mean of the C(λ)
values to the mean of the P(λ) values, as follows:
T
(
)
=
P
(
)
/n
C
(
)
/n
Where n ≥5
(e) Calculation of mean absorbance values.
(1) Mean transmittance values,
T
(
)
are converted into mean absorbance values,
A
(
)
,
at each wavelength by taking the negative logarithm of the mean transmittance value as
follows:
A
(
)
= log T
(
)
(2) The calculation yields 111 monochromatic absorbance values in 1 nanometer
increments from 290 to 400 nanometers.
(f) Number of plates. For each sunscreen product, determine mean absorbance values from at
least three individual PMMA plates. Because § M020.90(d) requires at least 5 measurements per
plate, there must be a total of at least 15 measurements.
(g) Calculation of the critical wavelength. The critical wavelength is identified as the
wavelength at which the integral of the spectral absorbance curve reaches 90 percent of the
integral over the UV spectrum from 290 to 400 nm. The following equation defines the critical
wavelength:
A
(
)
d
= 0.9 A
(
)
d
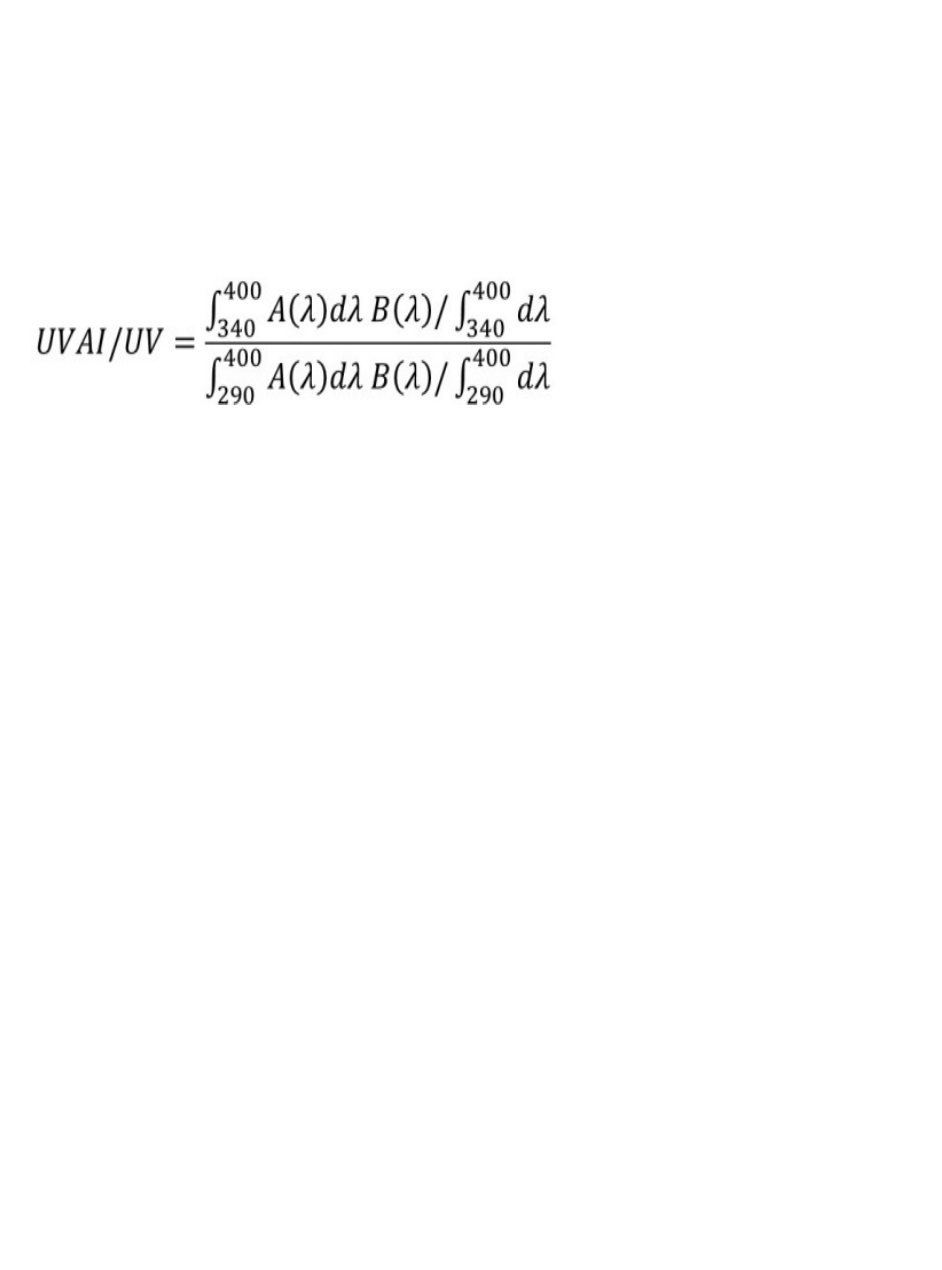
Proposed Order OTC000008
Page 127
Where λc = critical wavelength
A(λ) = mean absorbance at each wavelength
dλ = wavelength interval between measurements
(h) Calculation of the UVA I/UV ratio. The ratio of UVA I/UV is calculated as the area (per unit
wavelength) under the UVA I portions of a plot of wavelength versus A(λ), divided by the area
(per unit wavelength) under the total curve, as follows:
where:
A(λ) = effective absorbance given as -log T(λ)mean absorbance at each wavelength,
d(λ) = wavelength interval between measurements
B(λ) = any biological action spectrum factor
Because no appropriate biological action spectrum for UVA radiation damage has been
universally accepted, no action spectrum is specified. The value of B(λ) is, therefore,
equal to 1.0 for all wavelengths.
(i) Determination of broad spectrum protection. A product that has both a mean critical
wavelength of 370 nm or greater, calculated in accordance with § M020.90(g), and a mean UVA
I/UV ratio of 0.70 or greater, calculated in accordance with § M020.90(h), is determined to pass
the broad spectrum test.
§ M020.100 Regulatory status of final formulation testing and related requirements
Final formulation testing required under this OTC monograph is considered a part of the
manufacture of a sunscreen product. Therefore, final formulation testing required under this
OTC monograph must be performed in an establishment registered in accordance with 21 CFR
part 207 and section 510 of the FD&C Act (21 U.S.C. 360). Entities conducting final
formulation testing required by this OTC monograph must also comply with current good
manufacturing practices (CGMPs) and associated recordkeeping requirements including those
set forth in § M020.110 and in 21 CFR parts 210 and 211.
§ M020.110 Recordkeeping
Records required to be kept under this OTC monograph must be maintained for at least 1 year
after the expiration date of all products labeled in reliance on that testing or, in the case of certain
OTC drug products lacking expiration dating because they meet the criteria for exemption under
21 CFR 211.137, 3 years after distribution of the last lot of drug product bearing labeling that
relies on the testing. Recordkeeping requirements under this OTC monograph may not be
transferred. Maintenance records required to be kept under § M020.110(a) must be kept by the

Proposed Order OTC000008
Page 128
testing entity. Records of final formulation testing as described in §§ M020.110(b) and (c) must
be kept by the responsible person and any entity that is performing final formulation testing
required by this OTC monograph on behalf of a responsible person pursuant to a transfer of
obligations.
(a) Maintenance records. Entities performing final formulation testing are expected to maintain
equipment in accordance with § M020.100 and, as applicable, 21 CFR parts 210 and 211.
Maintenance records must be kept for all equipment used for final formulation testing under this
OTC monograph and must include:
(1) Documentation that equipment has been maintained in accordance with established
written specifications as required by § M020.100 and 21 CFR parts 210 and 211; and
(2) Documentation of characterization of UV sources including:
(i) Record of emission spectrum, total irradiance, and percent of erythema-
effective radiation contribution required by §M020.80(b)(1);
(ii) Record of each periodic measurement required by § M020.80(b)(4) for each
solar simulator;
(iii) Record of each calibration, realignment, or change in components of each
solar simulator, or any changes to the broadband radiometer (or UV meter/dose
control system), required by § M020.80(b)(4); and
(iv) Record of each solar simulator output measurement required by §
M020.80(b)(2)(iii).
(b) SPF testing records. In addition to any records required to be kept pursuant to 21 CFR parts
210 and 211, records of SPF testing performed pursuant to § M020.80 must include:
(1) Identification of the entity that conducted the final formulation testing, including the
name and address of the establishment(s) at which testing was carried out;
(2) The sunscreen test product identifier and characterization of the formulation being
tested, including lot number, manufacture date, and expected SPF;
(3) Characterization of the SPF standard sunscreen required by § M020.80(c), including:
(i) Lot number;
(ii) Manufacturing date; and
(iii) Results of HPLC SPF standard assay that verify compliance with the
concentrations of padimate O and oxybenzone in the SPF standard.

Proposed Order OTC000008
Page 129
(4) Documentation linking any blinded samples with the product identifier.
(5) For each human subject, records of:
(i) The identification of the UV source used for testing on that subject, including
make, model, and serial number;
(ii) Initial and final individual MED for unprotected skin (MED
u
), and the identity
of the study personnel who determined that value;
(iii) Final MED for sunscreen test product protected skin (
tp
MED
p
), and the
identity of the study personnel who determined that value;
(iv) Final MED for SPF standard sunscreen protected skin (
ss
MED
p
), and the
identity of the study personnel who determined that value; and
(v) Individual SPF
i
values, including all valid test data and invalid test data for the
test product and for the SPF standard sunscreen, and the identity of the study
personnel who determined that value.
(6) Records of the mean and standard deviation from SPF
i
values, standard error, and
determined SPF value derived as set forth in § M020.80(g).
(7) Records for water resistance testing of pool temperature, air temperature, and relative
humidity as required by § M020.80(h).
(8) Records demonstrating compliance with § M020.80(a) governing the establishment of
adequate clinical testing procedures and conditions, including, but not limited to:
(i) Case histories. Responsible persons are required to prepare and maintain
adequate and accurate case histories on each individual participant enrolled in
SPF testing performed under § M020.80. Case histories must record all
observations and other data pertinent to the investigation. Case histories include
the case report forms and supporting data (for example, signed and dated consent
forms, medical records including progress notes of the physician, the individual’s
hospital chart(s), and the nurses’ notes (if applicable)). The case history for each
individual participant must document that informed consent was obtained
pursuant to 21 CFR part 50 before each individual’s participation in the study.
Case histories as required by § M020.110 must include:
(A) Protocol deviations or injuries, if any; and
(B) Identification, by subject, of the study personnel who: examined the
potential study site areas, who weighed and applied the sunscreen, and
who provided the UV irradiation.

Proposed Order OTC000008
Page 130
(ii) IRB review. Documentation that clinical research conducted pursuant to §
M020.80 was reviewed and approved by a registered IRB as required by §
M020.80(a)(3).
(c) Broad spectrum testing records. Records of broad spectrum testing conducted pursuant to §
M020.90 must include:
(1) Identification of the entity that conducted the final formulation testing, including the
name and address of the establishment(s) at which testing was carried out;
(2) Records of sample information, including:
(i) A sunscreen test product identifier and expected SPF. If the samples used in
testing under § M020.90 are blinded, then records must include a master key that
enables samples to be re-identified. In all other cases, records must include a
master key that links samples used to a sunscreen test product identifier.
(ii) Sample number;
(iii) Identifier code;
(iv) Measurement of PMMA plate surface topography in micrometers; and
(v) Sample holder orientation (vertical or horizontal).
(3) Identification of each UV source used for sunscreen product pre-irradiation, including
make, model, and serial number.
(4) Records of sunscreen product application, including:
(i) A record of all sample weights, including analytical balance; and
(ii) For all equipment used; make, model, and serial number;
(5) For each sample, all measurements required by §§ M020.90(d) to (f).
(6) For each sample, records of critical wavelength and the UVA I/UV ratio values
required by §§ M020.90(g) and (h).
(7) For each sample: the identity of the study personnel who weighed and applied the
sunscreen to the PMMA plates; the identity of the study personnel who provided the pre-
irradiation; and the identity of the study personnel, or, if calculated by software, what
software, calculated the mean transmittance, mean absorbance values, critical
wavelength, and UVA I/UV.

Proposed Order OTC000008
Page 131
(8) For each sample, the test dates for the broad spectrum test conducted pursuant to §
M020.90, and sample report forms and supporting data including, for example, spectral
data, Excel files containing transmittance or absorbance values, or any notes from the lab
investigator.
(d) Food and Drug Administration inspection of records.
(1) Testing entity. An entity performing final formulation testing under this OTC
monograph, including a responsible person or an entity that has been transferred any
obligations of a responsible person under this OTC monograph, must, upon request from
any properly authorized officer or employee of FDA, at reasonable times, permit such
officer or employee to have access to, and copy and verify any records or reports of
testing pursuant to this OTC monograph. The testing entity is not required to divulge
subject names unless the records of particular individuals require a more detailed study of
the cases, or unless there is reason to believe that the records do not represent actual case
studies, or do not represent actual results obtained.
(2) Responsible persons. A responsible person must upon request from any properly
authorized officer or employee of FDA, at reasonable times, permit such officer or
employee to have access to and copy and verify any records and reports relating to final
formulation testing conducted under this OTC monograph. Upon written request by
FDA, the responsible person must submit the records or reports (or copies of them) to
FDA. The responsible person must discontinue from further participation in final
formulation testing required by this OTC monograph any investigator who has failed to
maintain or make available records or reports of the investigation as required by §
M020.110.

Proposed Order OTC000008
Page 132
We propose to amend the OTC Monograph M016: Skin Protectant Drug Products for
OTC Human Use, as follows:
U.S. Food and Drug Administration
Over-the-Counter Monograph M016:
Skin Protectant Drug Products for Over-the-Counter Human Use
1. Revise § M016.20(e) to read as follows:
* * * * *
§ M016.20
(e) Combinations of skin protectant and sunscreen active ingredients. Any one (two when
required to be in combination) or more of the skin protectant active ingredients identified in §§
M016.10(a), (d), (e), (g), (h), (i), (k), (l), (m), and (r) of this OTC monograph may be combined
with any single sunscreen active ingredient identified in § M020.10 of OTC monograph M020,
or any permitted combination of these ingredients identified in § M020.20(a), provided the
product meets the conditions in § M020.20(b).
2. Amend § M016.50 by revising paragraph (b) to read as follows:
(b) Indications. The labeling of the product states, under the heading "Uses," any of the phrases
listed in § M016.50(b), as appropriate. Other truthful and nonmisleading statements describing
only the indications for use that have been established and listed in § M016.50(b) or uses
permitted by § M016.60(b) for products containing both skin protectant and sunscreen active
ingredients, may also be used, as provided in 21 CFR 330.1(c)(2).
* * *
3. Amend § M016.60 by revising paragraphs (a), (b)(3), (c)(1), and (d)(1) to read as
follows:
§ M016.60 Labeling of permitted combinations of active ingredients
* * * * *
(a) Statement of identity.
(1) Except as set forth in § M016.60(a)(3), for a combination drug product that has an
established name, the labeling of the product states the established name of the
combination drug product, followed by the statement of identity for each ingredient in the
combination, as established in the statement of identity sections of the applicable OTC
monographs.
Proposed Order OTC000008
Page 133
(2) Except as set forth in § M016.60(a)(3), for a combination drug product that does not
have an established name, the labeling of the product states the statement of identity for
each ingredient in the combination, as established in the statement of identity sections of
the applicable OTC monographs.
(3) For a product containing a combination of skin protectant and sunscreen active
ingredients, the labeling of the product bears the statement of identity set forth in §
M020.60(a) of OTC Monograph M020.
(b) Indications. The labeling of the product states, under the heading "Uses," the indication(s) for
each ingredient in the combination as established in the indications sections of the applicable
OTC monographs, unless otherwise stated in § M016.60(b). Other truthful and nonmisleading
statements, describing only the indications for use that have been established in the applicable
OTC monographs or listed in § M016.60(b) may also be used, as provided in 21 CFR
330.1(c)(2). In addition to the required information identified in § M016.60(b), the labeling of
the product may contain any of the "other allowable statements" that are identified in the
applicable OTC monographs, provided such statements are neither placed in direct conjunction
with information required to appear in the labeling nor occupy labeling space with greater
prominence or conspicuousness than the required information.
* * *
(3) Combinations of skin protectant and sunscreen active ingredients in § M016.20(e). In
addition to any or all of the indications for skin protectant drug products in
§ M016.50(b)(2), the required indications for sunscreen drug products in § M020.52(a)(1)
of OTC Monograph M020 must be used. Any other indication in § M020.52(a) may also
be included if the product satisfies requirements for bearing that indication under that
section.
(c) * * *
(1) For combinations containing a skin protectant and a sunscreen identified in §
M016.20(e) and in § M020.20(b) of OTC Monograph M020. The labeling of the product
states, under the heading “Warnings,” the applicable warnings for sunscreens in §
M020.52(b) of OTC Monograph M020 and for skin protectants in § M016.50(c).
* * * * *
(d) * * *
(1) For combinations containing a skin protectant and a sunscreen identified in §
M016.20(e) and in § M020.20(b) of OTC Monograph M020. The labeling of the
product states, under the heading “Directions,” any or all of the applicable directions for
sunscreens, as set forth in § M020.52(c), and for skin protectants, as set forth in §
M016.50(d), unless otherwise stated in § M016.60(d). When the time intervals or age
limitations for administration of the individual ingredients differ, the directions for the
product may not contain any dosage that exceeds those established for any individual
Proposed Order OTC000008
Page 134
ingredient in the applicable OTC monograph(s), and may not provide for use by any age
group lower than the highest minimum age limit established for any individual
ingredient. When the directions for administration of the sunscreen and skin protectant
differ in any other way, the directions for sunscreens in § M020.52(c) should be used.
* * * * *
We propose to amend Non-Monograph Conditions NM900: Drug Products Containing
Certain Active Ingredients Offered Over-the-Counter for Certain Uses, as follows:
U.S. Food and Drug Administration
Non-Monograph Conditions NM 900:
Drug Products Containing Certain Active Ingredients Offered Over-the-Counter for
Certain Uses
1. Amend Non-Monograph Conditions NM900: Drug Products Containing Certain
Active Ingredients Offered Over-the-Counter for Certain Uses by removing paragraph (a)(24)
We propose to establish Non-Monograph Conditions NM020: Sunscreen Drug Products
for Over-the-Counter Use, containing the following provisions:
1. Add new administrative order NM020 to read as follows:
U.S. Food and Drug Administration
Non-Monograph Conditions NM020:
Sunscreen Drug Products for Over-the-Counter Use
(Proposal Issued September 24, 2021)
Part A—General Provisions
Sec.
NM020.1 Scope
Part B—Specific Non-Monograph Conditions
NM020.3 Non-Monograph Active Ingredients
NM020.5 Non-Monograph SPF and Broad Spectrum Conditions
NM020.10 Non-Monograph Labeling and Marketing
NM020.20 Non-Monograph Dosage Form Conditions
Part A—General Provisions
§ NM020.1 Scope
Proposed Order OTC000008
Page 135
Any drug product offered OTC for use as sunscreen and identified in any provision of § NM020
is not generally recognized as safe and effective and is as a new drug within the meaning of
section 201(p) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 U.S.C 321(p)), for
which an approved new drug application under section 505 of the FD&C Act (21 U.S.C. 355(a))
and 21 CFR part 314 is required for marketing. In the absence of an approved new drug
application, such product is also misbranded under section 502 of the FD&C Act (21 U.S.C 352).
Products offered OTC for use as sunscreen include those represented, labeled, or promoted as
sunscreen, or for use to help prevent sunburn, skin cancer, and/or skin aging caused by the sun,
or with similar claims or representations. Clinical investigations designed to obtain evidence that
any sunscreen drug product covered by § NM020 is safe and effective for the purpose intended
must comply with the requirements and procedures governing the use of investigational new
drugs set forth in 21 CFR part 312.
Part B—Non-monograph Conditions
§ NM020.3 Non-Monograph Active Ingredients
A sunscreen drug product that contains any of the following active ingredients:
(a) Aminobenzoic acid
(b) Avobenzone
(c) Cinoxate
(d) Diethanolamine methoxycinnamate
(e) Digalloyl trioleate
(f) Dioxybenzone
(g) Ensulizole
(h) Ethyl 4-[bis(hydroxypropyl)] aminobenzoate
(i) Glyceryl aminobenzoate
(j) Homosalate
(k) Lawsone with dihydroxyacetone
(l) Meradimate
(m) Octinoxate
Proposed Order OTC000008
Page 136
(n) Octisalate
(o) Octocrylene
(p) Oxybenzone
(q) Padimate O
(r) Red petrolatum
(s) Sulisobenzone
(t) Trolamine salicylate
§ NM020.5 Non-Monograph SPF and Broad Spectrum Conditions
(a) A sunscreen drug product that has a determined sun protection factor (SPF) value, as defined
in § M020.3(b) of OTC Monograph M020, of at least 15 when tested in accordance with §
M020.80 of OTC Monograph M020, but that has not been shown to pass the broad spectrum test
in § M020.90 of OTC Monograph M020.
(b) A sunscreen drug product that has a determined sun protection factor (SPF) value, as defined
in § M020.3(b) of OTC Monograph M020, of less than 2 or greater than 80 when tested in
accordance with § M020.80 of OTC Monograph M020.
§ NM020.10 Non-Monograph Labeling and Marketing
(a) A sunscreen drug product that has a determined sun protection factor (SPF) value, as defined
in § M020.3(b) of OTC Monograph M020, of less than 15 when tested in accordance with §
M020.80 of OTC Monograph M020 and/or that does not pass the broad spectrum test in §
M020.90 of OTC Monograph M020, and labeled with any of the following or similar claims:
(1) Decreases the risk of skin cancer caused by the sun; or
(2) Decreases the risk of early skin aging caused by the sun.
(b) A sunscreen drug product labeled with any of the following or similar claims:
(1) Instant protection or protection immediately upon application; or
(2) Claims for “all-day” protection or extended wear claims citing a specific number of
hours of protection that is inconsistent with the directions for application in OTC
Monograph M020.
(c) A sunscreen drug product that is labeled, represented, or promoted for use as a combined
sunscreen-insect repellant.

Proposed Order OTC000008
Page 137
§ NM020.20 Non-Monograph Dosage Form Conditions
(a) A sunscreen drug product that is in any dosage form other than the following: oil, lotion,
cream, gel, butter, paste, ointment, stick, or spray.
(b) A sunscreen drug product in a spray dosage form that has any of the following properties:
(1) The product meets the definition of the term “extremely flammable” as defined at
§ M020.3(d) of OTC Monograph M020 when tested in accordance with 16 CFR
1500.43a, as published at 51 FR 28539 (August 8, 1986);
(2) More than 10 percent of the particles dispensed from the consumer container are
smaller than 10 micrometers;
(3) Any of the particles dispensed from the consumer container are smaller than 5
micrometers; or
(4) The product meets the definition of either the term “flammable” or the term
“combustible” as defined at §§ M020.3(e) or (f) of OTC Monograph M020, as applicable,
when tested in accordance with 16 CFR 1500.43a, as published at 51 FR 28539 (August
8, 1986), and has a measured drying time of 10 minutes or more.
VII. EXCLUSIVITY
This proposed order, if finalized, will not grant exclusivity to any entity.
VIII. PUBLIC COMMENT
Submit electronic comments on the proposed order by November 12, 2021, via
https:www.regulations.gov to docket no. FDA-1978-N-0018. See the corresponding Federal
Register Notice of Availability of this proposed order for instructions on how to submit
comments. As noted above, FDA will consider comments that were submitted to the docket for
the 2019 Proposed Rule within its comment period to be constructively submitted as comments
on this proposed order. As also noted above, to enable the Agency to review and address these
comments (and future comments that may be submitted on this proposed order) as expeditiously
as possible, we request that commenters do not re-submit the same comments previously
submitted on the proposed rule.
IX. ANALYSIS OF ENVIRONMENTAL IMPACTS
We have determined that this action may have potential impacts on the human
environment. Pursuant to the National Environmental Policy Act, the Agency has issued a Notice
of Intent to Prepare an Environmental Impact Statement for Certain Sunscreen Drug Products for

Proposed Order OTC000008
Page 138
Over-the-Counter Use (FDA-2021-N-0352) to evaluate potential environmental impacts
associated with the use of oxybenzone and octinoxate in sunscreens.
X. REFERENCES AND MATERIALS PROPOSED FOR
INCORPORATION BY REFERENCE
The following references and material proposed for incorporation by reference marked
with an asterisk (*) are on display at the Dockets Management Staff (HFA-305), Food and Drug
Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852, and are available for
viewing by interested persons between 9 a.m. and 4 p.m., Monday through Friday (240-402-
7500); they are also available electronically at http://www.regulations.gov. References and
material proposed for incorporation by reference in the order without asterisks are not available
electronically at https://www.regulations.gov because they have copyright restrictions, but they
are on display at the Dockets Management Staff and available for viewing at the location and
times noted above. Some may be available at the website address, if any, listed with the
reference or material proposed for incorporation by reference or such website may provide
further information on obtaining copies. FDA has verified the website addresses, as of the date
this document is posted, but websites are subject to change over time.
Adachi, K., N. Yamada, K. Yamamoto, et al., “In Vivo Effect of Industrial Titanium Dioxide
Nanoparticles Experimentally Exposed to Hairless Rat Skin,” Nanotoxicology, vol. 4(3), pp. 296-
306, 2010.
Adachi, K., N. Yamada, Y. Yoshida, et al., “Subchronic Exposure of Titanium Dioxide
Nanoparticles to Hairless Rat Skin,” Experimental Dermatology, vol. 22(4), pp. 278-283, 2013.
Agren, M.S., “Influence of Two Vehicles for Zinc Oxide on Zinc Absorption through Intact Skin
and Wounds,” Acta Dermato-Venereologica, vol. 71(2), pp. 153-156, 1991.
Agren, M.S., “Percutaneous Absorption of Zinc from Zinc Oxide Applied Topically to Intact
Skin in Man,” Dermatologica, vol. 180(1), pp. 36-39, 1990a.
Agren, M.S., “Studies on Zinc in Wound Healing,” Acta Dermato-Venereologica.
Supplementum, vol. 154, pp. 1-36, 1990b.
Alexopoulos, E., E. Papagianni, M. Leontsini, et al., “Para-Amino-Benzoic Acid (PABA) and
Chronic Tubulointerstitial Nephritis,” Clinical Nephrology, vol. 40(6), pp. 359-360, 1993.
American Cancer Society, “Melanoma Skin Cancer” (available at
https://www.cancer.org/cancer/melanoma-skin-
cancer.html?gclid=CMCkj93Uqs4CFYtahgodxIwIYA&mkwid=s_dc;
https://www.cancer.org/cancer/melanoma-skin-cancer.html), accessed May 25, 2021.

Proposed Order OTC000008
Page 139
American Academy of Pediatrics, “Tips for Using Repellents Safely” (available at
www.healthychildren.org/English/safety-prevention/at-play/Pages/Insect-Repellents.aspx?rf),
accessed May 25, 2021.
Ang, P., S.K. Ng, and C.L. Goh, “Sunscreen Allergy in Singapore,” American Journal of
Contact Dermatitis, vol. 9(1), pp. 42-44, 1998.
Autier, P., M. Boniol, and J.F. Dore, “Sunscreen Use and Increased Duration of Intentional Sun
Exposure: Still a Burning Issue,” International Journal of Cancer, vol. 121(1), pp. 1-5, 2007.
Balogh, T.S., M.V. Velasco, C.A. Pedriali, et al., “Ultraviolet Radiation Protection: Current
Available Resources in Photoprotection,” Anais Brasileiros de Dermatologia, vol. 86(4), pp.
732-742, 2011.
Bashaw, E.D, D.C. Tran, C.G. Shukla, et al., “Maximal Usage Trial: An Overview of the Design
of Systemic Bioavailability Trial for Topical Dermatological Products,” Therapeutic Innovation
and Regulatory Science, vol. 49(1), pp. 108-115, 2015.
Bassani, A.S., D. Banov, and H. Phan, “Characterization of the Percutaneous Absorption of
Ketoprofen Using the Franz Skin Finite Dose Model,” Postgraduate Medical Journal, vol.
128(2), pp. 262-267, 2017.
Beasley, D.G. and T.A. Meyer, “Characterization of the UVA Protection Provided by
Avobenzone, Zinc Oxide, and Titanium Dioxide in Broad-Spectrum Sunscreen Products,”
American Journal of Clinical Dermatology, vol. 11(6), pp. 413-421, 2010.
Beeckman, D., L. Schoonhoven, S. Verhaeghe, et al., “Prevention and Treatment of
Incontinence-Associated Dermatitis: Literature Review,” Journal of Advanced Nursing, vol.
65(6), pp. 1141-1154, 2009.
Benson, H.A., V. Sarveiya, S. Risk, et al., “Influence of Anatomical Site and Topical
Formulation on Skin Penetration of Sunscreens,” Therapeutics and Clinical Risk Management,
vol. 1(3), pp. 209-218, 2005.
Borum, M., E. Nsien, and H. Zimmerman, “Hepatotoxicity from Paraaminobenzoic Acid,”
Digestive Diseases and Sciences, vol. 36(12), pp. 1793, 1991.
Brash, D.E., “UV-Induced Melanin Chemiexcitation: A New Mode of Melanoma Pathogenesis,”
Toxicologic Pathology, vol. 44(4), pp. 552-554, 2016.
Brinon, L., S. Geiger, V. Alard, et al., “Percutaneous Absorption of Sunscreens from Liquid
Crystalline Phases,” Journal of Controlled Release, vol. 60(1), pp. 67-76, 1999.
Brown, S. and B.L. Diffey, “The Effect of Applied Thickness on Sunscreen Protection: In Vitro
and In Vivo Studies,” Photochemistry and Photobiology, vol. 44(4), pp. 509-13, 1986.

Proposed Order OTC000008
Page 140
Brown, J.S., T. Gordon, O. Price, et al., “Thoracic and Respirable Particle Definitions for Human
Health Risk Assessment,” Particle and Fibre Toxicology, vol. 10, p. 12, 2013
Bruynzeel, D.P., J. Ferguson, K. Andersen, et al., “Photopatch Testing: A Consensus
Methodology for Europe,” Journal of the European Academy of Dermatology and Venereology,
vol. 18(6), pp. 679-682, 2004.
Bryden, A.M., H. Moseley, S.H. Ibbotson, et al., “Photopatch Testing of 1155 Patients: Results
of the U.K. Multicentre Photopatch Study Group,” British Journal of Dermatology, vol. 155(4),
pp. 737-747, 2006.
Burnett, M.E. and S.Q. Wang, “Current Sunscreen Controversies: A Critical Review,”
Photodermatology, Photoimmunology and Photomedicine, vol. 27(2), pp. 58-67, 2011.
Calafat, A.M., L.Y. Wong, X. Ye, et al., “Concentrations of the Sunscreen Agent Benzophenone-
3 in Residents of the United States: National Health and Nutrition Examination Survey 2003-
2004,” Environmental Health Perspectives, vol. 116(7), pp. 893-897, 2008.
*CDC, Travelers’ Health, Chapter 2: The Pretravel Consultation. J. Mutebi, W.A. Hawley, and
W.G. Brogdon, “Protection against Mosquitoes, Ticks, and Other Arthropods” (available at
https://wwwnc.cdc.gov/travel/yellowbook/2018/the-pre-travel-consultation/protection-against-
mosquitoes-ticks-other-arthropods), accessed May 25, 2021.
*Centers for Disease Control and Prevention (CDC), “Skin Cancer: Sun Safety” (available at
https://www.cdc.gov/cancer/skin/basic_info/sun-safety.htm), accessed May 25. 2021
Ceresole, R., Y.K. Han, M.A. Rosasco, et al., “Drug-Excipient Compatibility Studies in Binary
Mixtures of Avobenzone,” Journal of Cosmetic Science, vol. 64(5), pp. 317-328, 2013.
Chao, L.X., S.L. Sheu, B.Y. Kong, et al. “Identifying Gaps in Consumer Knowledge About
Sunscreen,” Journal of the American Academy of Dermatology, vol. 77(6), pp. 1172-1173. 2017.
Coelho, S.G., Y. Zhou, H.F. Bushar, et al., “Long-Lasting Pigmentation of Human Skin, A New
Look at an Overlooked Response to UV,” Pigment Cell and Melanoma Research, vol. 22(2), pp.
238-241, 2009.
Crosera, M., A. Prodi, M. Mauro, et al., “Titanium Dioxide Nanoparticle Penetration into the
Skin and Effects on HaCaT Cells,” International Journal of Environmental Research and Public
Health, vol. 12(8), pp. 9282-9297, 2015.
Damian, D.L., Y.J. Matthews, T.A. Phan, et al., “An Action Spectrum for Ultraviolet Radiation-
Induced Immunosuppression in Humans,” British Journal of Dermatology, vol. 164(3), pp. 657-
659, 2011.

Proposed Order OTC000008
Page 141
Darvin, M.E., K. Konig, M. Kellner-Hoefer, et al., “Safety Assessment by Multiphoton
Fluorescence/Second Harmonic Generation/Hyper-Rayleigh Scattering Tomography of ZnO
Nanoparticles Used in Cosmetic Products,” Skin Pharmacology and Physiology, vol. 25(4), pp.
219-226, 2012.
Diffey, B., “The FDA Final Rule on Labeling and Effectiveness Testing of Sunscreens: Too
Little, Too Late?” Journal of the American Academy of Dermatology, vol. 66(1), pp. 162-163,
2012.
Diffey, B., “Spectral Uniformity: A New Index of Broad Spectrum (UVA) Protection,”
International Journal of Cosmetic Science, vol. 31(1), pp. 63-68, 2009.
Diffey, B.L., “Sunscreens and UVA Protection: A Major Issue of Minor Importance,”
Photochemistry and Photobiology, vol. 74(1), pp. 61-63, 2001.
DiGiovanna, J.J. and K.H. Kraemer, “Shining a Light on Xeroderma Pigmentosum,” Journal of
Investigative Dermatology, vol. 132(3), pp. 785-796, 2012.
Dromgoole, S.H. and H.I. Maibach, “Sunscreening Agent Intolerance: Contact and Photocontact
Sensitization and Contact Urticaria,” Journal of the American Academy of Dermatology, vol.
22(6 Pt 1), pp. 1068-1078, 1990.
Environmental Working Group, “CDC: Americans Carry Body Burden of Toxic Sunscreen
Chemical,” March 2008 (available at https://www.ewg.org/research/cdc-americans-carry-body-
burden-toxic-sunscreen-chemical#.WrpKP8a1tPZ), accessed May 25, 2021..
*EPA, “Active Ingredients Eligible for Minimum Risk Pesticide Products” (Updated December
2015) (available at https://www.epa.gov/sites/production/files/2015-12/documents/minrisk-
active-ingredients-tolerances-2015-12-15.pdf), accessed May 25, 2021.
*EPA, Office of Pesticide Programs, Label Review Manual, “Chapter 3: General Labeling
Requirements,” revised March 2018 (available at
https://www.epa.gov/sites/production/files/2017-10/documents/chap-03-dec-2014.pdf
).),
accessed May 25, 2021.
EPA, “DEET” (available at https://www.epa.gov/insect-repellents/deet#children), accessed May
25, 2021.
*EPA, “Find the Repellent That is Right for You,” (Updated June 2019) (available at
https://www.epa.gov/insect-repellents/find-repellent-right-you), accessed May 25, 2021.
* “Reregistration Eligibility Decision (R.E.D.) Facts, DEET,” April 1998 (available at
https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/fs_PC-080301_1-Apr-
98.pdf), accessed June 7, 2021.

Proposed Order OTC000008
Page 142
*EPA, “Using Insect Repellents Safely and Effectively” (available at
https://www.epa.gov/insect-repellents/using-insect-repellents-safely-and-effectively), accessed
May 25, 2021.
*European Commission, “Commission Recommendation of 22 September 2006 on the Efficacy
of Sunscreen Products and the Claims Made Relating Thereto (2006/647/EC),” Official Journal
of the European Union, vol. 265, pp. 39-43, 2006 (available at http://eur-lex.europa.eu/legal-
content/EN/TXT/PDF/?uri=CELEX:32006H0647&from=EN), accessed May 25, 2021.
*European Commission. Scientific Committee on Consumer Products (SCCP), “Opinion on
Benzophenone-3,” 2008 (available at
https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_159.pdf), accessed May 25,
2021.
*European Commission, SCCNFP, “Opinion on the Evaluation of Potentially Estrogenic Effects
of UV-Filters Adopted by the SCCNFP During the 17th Plenary Meeting of 12 June 2001”
(available at
(http://ec.europa.eu/health/scientific_committees/consumer_safety/opinions/sccnfp_opinions_97
_04/sccp_out145_en.htm), accessed May 25, 2021.
*European Commission, Scientific Committee on Consumer Safety (SCCS), “Opinion on
Titanium Dioxide (nano form),” 2014c, revision (available at
http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_136.pdf
),
accessed May 25, 2021.
*European Commission, SCCS, “Opinion on Zinc Oxide (nano form),” 2012 (available at
http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_103.pdf
),
accessed April 4, 2018.
European Multicentre Photopatch Test Study (EMCPPTS) Taskforce, “A European Multicentre
Photopatch Test Study,” The British Journal of Dermatology, vol. 166(5), pp. 1002-1009, 2012.
Fairhurst, D. and M.A. Mitchnick, “Particulate Sun Blocks: General Principles,” N.J. Lowe,
N.A. Shaath, M.A. Pathak, editors, Sunscreens: Development, Evaluation, and Regulatory
Aspects, 2nd ed., New York: M. Dekker, pp. 313-352, 1997.
Faurschou, A. and H.C. Wulf, “Synergistic Effect of Broad-Spectrum Sunscreens and
Antihistamines in the Control of Idiopathic Solar Urticaria,” Archives of Dermatology, vol.
144(6), pp. 765-769, 2008.
FDA, Guidance for Industry, “Carcinogenicity Study Protocol Submissions,” May 2002
(available at available at https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-
gen/documents/document/ucm078924.pdf), accessed May 11, 2021.
*FDA, Guidance for Industry, “Considering Whether an FDA-Regulated Product Involves the
Application of Nanotechnology,” 2014a (available at

Proposed Order OTC000008
Page 143
http://www.fda.gov/RegulatoryInformation/Guidances/ucm257698.htm), accessed May 25,
2021.
*FDA, Guidance for Industry, “Enforcement Policy--OTC Sunscreen Drug Products Marketed
Without an Approved Application,” 2018a (available at
https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM25900
1), accessed May 25, 2021.
*FDA, “Inactive Ingredient Search for Approved Drug Products” (available at
https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm), accessed May 25, 2021.
*FDA, Guidance for Industry, “M7(R1) Assessment and Control of DNA Reactive (Mutagenic)
Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk,” 2018b (available at
https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/
UCM347725.pdf), accessed May 25, 2021.
*FDA, Guidance for Industry, “Maximal Usage Trials for Topically Applied Active Ingredients
Being Considered for Inclusion in an Over-The-Counter Monograph: Study Elements and
Considerations,” 2019a (available at
https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-
drugs-gen/documents/document/ucm608356.pdf), accessed May 25, 2021.
*FDA, Guidance for Industry, “The Need for Long-term Rodent Carcinogenicity Studies of
Pharmaceuticals,” March 1996 (available at https://www.fda.gov/ucm/groups/fdagov-
public/@fdagov-drugs-gen/documents/document/ucm074911.pdf), accessed May 25, 2021.
*FDA, Guidance for Industry, “Nonclinical Evaluation of Endocrine-Related Drug Toxicity,”
2015a (available at https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-
gen/documents/document/ucm369043.pdf), accessed May 25, 2021.
*FDA, “Nonprescription Drug Advisory Committee, September 4-5, 2014, Meeting Minutes,”
2014b (available at
https://wayback.archive-
it.org/7993/20170404152726/https://www.fda.gov/downloads/AdvisoryCommittees/Committees
MeetingMaterials/Drugs/NonprescriptionDrugsAdvisoryCommittee/UCM421304.pdf), accessed
May 25, 2021.
*FDA, Guidance for Industry, “Nonprescription Sunscreen Drug Products--Safety and
Effectiveness Data,” November 2016 (available at
https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/
UCM473464.pdf), accessed May 25, 2018.
*FDA, Guidance for Industry, “S5A Detection of Toxicity to Reproduction for Medicinal
Products,” September 1994 (available at
https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/u
cm074950.pdf), accessed May 25, 2021.

Proposed Order OTC000008
Page 144
FDA, Guidance for Industry, “S5(R3) Detection of Reproductive and Developmental Toxicity
for Human Pharmaceuticals,” May 2021 (available at https://www.fda.gov/regulatory-
information/search-fda-guidance-documents/s5r3-detection-reproductive-and-developmental-
toxicity-human-pharmaceuticals), accessed July 1, 2021.
*FDA, Guidance for Industry, “S10 Photosafety Evaluation of Pharmaceuticals,” 2015b
(available at https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-
gen/documents/document/ucm337572.pdf), accessed May 25, 2021.
*FDA, “Sunscreen: How to Help Protect Your Skin from the Sun,” 2019b (available at
https://www.fda.gov/drugs/resourcesforyou/consumers/buyingusingmedicinesafely/understandin
gover-the-countermedicines/ucm239463.htm), accessed May 25, 2021.
*FDA, Preliminary Regulatory Impact Analysis, “Sunscreen Drug Product for Over-the-Counter
Human Use; Proposal to Amend and Lift Stay on Monograph” 2019 (available at
https://www.fda.gov/AboutFDA/ReportsManualsForms/Reports/EconomicAnalyses/default.htm,
accessed May 25, 2021.
*FDA, Guidance for Industry, “Toxicokinetics: The Assessment of Systemic Exposure in
Toxicity Studies,” March 1995 (available at
https://www.fda.gov/ucm/groups/fdagov-
public/@fdagov-drugs-gen/documents/document/ucm074937.pdf), accessed May 25, 2021.
*FDA, “Use Sunscreen Spray? Avoid Open Flame,” July 3, 2013 (available at
http://wayback.archive-
it.org/7993/20180125094109/https:/www.fda.gov/ForConsumers/ConsumerUpdates/ucm359437.
htm), accessed May 25, 2021.
Fisher, A.A., “Sunscreen Dermatitis: Para-Aminobenzoic Acid and its Derivatives,” Cutis, vol.
50(3), pp. 190-192, 1992.
Fourtanier, A., D. Moyal, and S. Seite, “Sunscreens Containing the Broad-Spectrum UVA
Absorber, Mexoryl SX, Prevent the Cutaneous Detrimental Effects of UV Exposure: A Review
of Clinical Study Results,” Photodermatology, Photoimmunology & Photomedicine, vol. 24(4),
pp. 164-174, 2008.
Franz, T.J., P.A. Lehman, and S.G. Raney, “Use of Excised Human Skin to Assess the
Bioequivalence of Topical Products,” Skin Pharmacology and Physiology, vol. 22, pp. 276-286,
2009.
Freitas, J.V., F.S. Praca, M.V. Bentley, et al., “Trans-Resveratrol and Beta-Carotene from
Sunscreens Penetrate Viable Skin Layers and Reduce Cutaneous Penetration of UV-Filters,”
International Journal of Pharmaceutics, vol. 48(1-2), pp. 131-137, 2015.
French, J.E., “NTP Technical Report on the Toxicity Studies of 2-Hydroxy-4-
Methoxybenzophenone (Cas No. 131-57-7) Adminstered Topically and in Dosed Feed to F344/N
Rats and B6c3f1 Mice,” Toxicity Report Series, vol. 21, pp. 1-e14, 1992.
Proposed Order OTC000008
Page 145
Fujishima, A., T.N. Rao, and D.A. Tryk, “Titanium dioxide photocatalysis,” Journal of
Photochemistry and Photobiology C: Photochemistry Reviews, vol. 1(1), pp. 1-21, 2000.
Gad, S.C., “Titanium.” P. Wexler, editors, Encyclopedia of Toxicology (Third Edition), Oxford:
Academic Press, 2014: 584-585.
Gao, L., Y. Hu, C. Ni, et al., “Retrospective Study of Photopatch Testing in a Chinese Population
During a 7-Year Period,” Dermatitis, vol. 25(1), pp. 22-26, 2014.
Girschik, J., L. Fritschi, T. Threlfall, et al., “Deaths from Non-Melanoma Skin Cancer in
Western Australia,” Cancer Causes and Control, vol. 19, pp. 879-885, 2008.
Golmohammadzadeh, S., M.R. Jaafarixx, and N. Khalili, “Evaluation of Liposomal and
Conventional Formulations of Octyl Methoxycinnamate on Human Percutaneous Absorption
Using the Stripping Method,” Journal of Cosmetic Science, vol. 59(5), pp. 385-398, 2008.
Gomez, E., A. Pillon, H. Fenet, et al., “Estrogenic Activity of Cosmetic Components in Reporter
Cell Lines: Parabens, UV Screens, and Musks,” Journal of Toxicology and Environmental
Health, vol. 68(4), pp. 239-251, 2005.
Gotman, I., “Characteristics of Metals Used in Implants,” Journal of Endourology, vol. 11(6),
pp. 383-389, 2009.
Gu, X., T. Wang, D.M. Collins, et al., “In Vitro Evaluation of Concurrent Use of Commercially
Available Insect Repellent and Sunscreen Preparations,” British Journal of Dermatology, vol.
152(6), pp. 1263-1267, 2005.
Gulson, B., H. Wong, M. Korsch, et al., “Comparison of dermal absorption of zinc from different
sunscreen formulations and differing UV exposure based on stable isotope tracing,” The Science
of the Total Environment, vol. 420, pp. 313-318, 2012.
Gulson, B., M. McCall, M. Korsch, et al., “Small amounts of zinc from zinc oxide particles in
sunscreens applied outdoors are absorbed through human skin,” Toxicological Sciences, vol.
118(1), pp. 140-149, 2010.
Gupta, V.K., J.L. Zatz, and M. Rerek, “Percutaneous Absorption of Sunscreens through Micro-
Yucatan Pig Skin in Vitro,” Pharmaceutical Research, vol. 16(10), pp. 1602-1607, 1999.
Gustavsson Gonzalez, H., A. Farbrot, and O. Larko, “Percutaneous Absorption of
Benzophenone-3, a Common Component of Topical Sunscreens,” Clinical and Experimental
Dermatology, vol. 27(8), pp. 691-694, 2002.
Hanson, K.M., E. Gratton, and C.J. Bardeen, “Sunscreen Enhancement of UV-Induced Reactive
Oxygen Species in the Skin,” Free Radical Biology and Medicine, vol. 41(8), pp. 1205-1212,
2006.

Proposed Order OTC000008
Page 146
Hayden, D.R., A. Imhof, and K.P. Velikov, “Biobased Nanoparticles for Broadband UV
Protection with Photostabilized UV Filters,” American Chemical Society Applied Materials and
Interfaces, vol. 8(48), pp. 32655-32660, 2016.
Hayden, C.G., S.E. Cross, C. Anderson, et al., “Sunscreen Penetration of Human Skin and
Related Keratinocyte Toxicity after Topical Application,” Skin Pharmacology and Physiology,
vol. 18(4), pp. 170-174, 2005.
Heurung, A.R., S.I. Raju, and E.M. Warshaw, “Adverse Reactions to Sunscreen Agents:
Epidemiology, Responsible Irritants and Allergens, Clinical Characteristics, and Management,”
Dermatitis, vol. 25(6), pp. 289-326, 2014a.
Heurung, A.R., S.I. Raju, and E.M. Warshaw, “Benzophenones,” Dermatitis, vol. 25(1), pp. 3-
10, 2014b.
Hodis, E., I.R. Watson, G.V. Kryukov, et al., “A Landscape of Driver Mutations in Melanoma,”
Cell, vol. 150(2), pp. 251-263, 2012.
Hong, J.S., M.K. Park, M.S. Kim, et al., “Effect of Zinc Oxide Nanoparticles on Dams and
Embryo-Fetal Development in Rats,” International Journal of Nanomedicine, vol. 9 Suppl 2, pp.
145-157, 2014a.
Hong, J.S., M.K. Park, M.S. Kim, et al., “Prenatal Development Toxicity Study of Zinc Oxide
Nanoparticles in Rats,” International Journal of Nanomedicine, vol. 9 Suppl 2, pp. 159-171,
2014b.
Ipach, I., R. Schafer, F. Mittag, et al., “The Development of Whole Blood Titanium Levels after
Instrumented Spinal Fusion--Is There a Correlation between the Number of Fused Segments and
Titanium Levels?,” BioMed Central Musculoskeletal Disorders, vol. 13, pp. 159, 2012.
ISO/CIE 17166:2019(E), “Erythema Reference Action Spectrum and Standard Erythema Dose”
(First Edition), May 2019 (available at https://www.iso.org/standard/74167.html), accessed July
27, 2021.
Jacobs, A.C. and P.C. Brown, “Regulatory Forum Opinion Piece; Transgenic/Alternative
Carcinogenicity Assays: A Retrospective Review of Studies Submitted to CDER/FDA 1997-
2014,” Toxicology Pathology, vol. 43(5), pp. 605-610, 2015.
Janjua, N.R., B. Kongshoj, A.M. Andersson, et al., “Sunscreens in Human Plasma and Urine
after Repeated Whole-Body Topical Application,” Journal of the European Academy of
Dermatology and Venereology, vol. 22(4), pp. 456-461, 2008.
Janjua, N.R., B. Kongshoj, J.H. Petersen, et al., “Sunscreens and Thyroid Function in Humans
after Short-Term Whole-Body Topical Application: A Single-Blinded Study,” The British
Journal of Dermatology, vol. 156(5), pp. 1080-1082, 2007.
Proposed Order OTC000008
Page 147
Janjua, N.R., B. Mogensen, A.M. Andersson, et al., “Systemic Absorption of the Sunscreens
Benzophenone-3, Octyl-Methoxycinnamate, and 3-(4-Methyl-Benzylidene) Camphor after
Whole-Body Topical Application and Reproductive Hormone Levels in Humans,” The Journal
of Investigative Dermatology, vol. 123(1), pp. 57-61, 2004.
Jiang, R., M.S. Roberts, R.J. Prankerd, et al., “Percutaneous Absorption of Sunscreen Agents
from Liquid Paraffin: Self-Association of Octyl Salicylate and Effects on Skin Flux,” Journal of
Pharmaceutical Sciences, vol. 86(7), pp. 791-796, 1997.
Journe, F., M.C. Marguery, J. Rakotondrazafy, et al., “Sunscreen Sensitization: A 5-Year
Study,” Acta Dermato-Venereologica, vol. 79(3), pp. 211-213, 1999.
Kadry, A.M., C.S. Okereke, M.S. Abdel-Rahman, et al., “Pharmacokinetics of Benzophenone-3
after Oral Exposure in Male Rats,” Journal of Applied Toxicology, vol. 15(2), pp. 97-102, 1995.
Kaldor, J., D. Shugg, B. Young, et al., “Non-Melanoma Skin Cancer: Ten Years of Cancer-
Registry-Based Surveillance,” International Journal of Cancer, vol. 53, pp. 886-891, 1993.
Kasichayanula S., J.D. House, T. Wang, et al., “Percutaneous Characterization of the Insect
Repellent DEET and the Sunscreen Oxybenzone from Topical Skin Application,” Toxicology
and Applied Pharmacology, vol. 223(2), pp. 187-194, 2007.
Kasichayanula, S., J.D. House, T. Wang, et al., “Simultaneous Analysis of Insect Repellent
DEET, Sunscreen Oxybenzone and Five Relevant Metabolites by Reversed-Phase HPLC with
UV Detection: Application to an In Vivo Study in a Piglet Model,” Journal of Chromatography
B. Analytical Technologies in the Biomedical and Life Sciences, vol. 882(1-2), pp. 271-277,
2005.
Kiss, B., T. Biro, G. Czifra, et al., “Investigation of Micronized Titanium Dioxide Penetration in
Human Skin Xenografts and Its Effect on Cellular Functions of Human Skin-Derived Cells,”
Experimental Dermatology, vol. 17(8), pp. 659-667, 2008.
Kockler, J., S. Robertson, M. Oelgemoller, et al., “Chapter 3: Butyl Methoxy
Dibenzoylmethane.” H. G. Brittian, editor. Profiles of Drug Substances, Excipients, and Related
Methodology, vol. 38, Elsevier, pp. 87-111, 2013.
Kong, B.Y., S.L. Sheu, and R.V. Kundu, “Assessment of Consumer Knowledge of New
Sunscreen Labels,” JAMA Dermatology, vol.151(9), pp. 1028-1030, 2015.
Krause, M., A. Klit, M. Blomberg Jensen, et al., “Sunscreens: Are They Beneficial for Health?
An Overview of Endocrine Disrupting Properties of UV-Filters,” International Journal of
Andrology, vol. 35(3), pp. 424-436, 2012.
Krauthammer, M., Y. Kong, B.H. Ha, et al., “Exome Sequencing Identifies Recurrent Somatic
Rac1 Mutations in Melanoma,” Nature Genetics, vol. 44(9), pp. 1006, 2012.

Proposed Order OTC000008
Page 148
Kuhn, A., K. Gensch, M. Haust, et al., “Photoprotective Effects of a Broad-Spectrum Sunscreen
in Ultraviolet-Induced Cutaneous Lupus Erythematosus: a Randomized, Vehicle-Controlled,
Double-Blind Study,” Journal of the American Academy of Dermatology, vol. 64(1), pp. 37-48,
2011.
Kurul, E. and S. Hekimoglu, “Skin Permeation of Two Different Benzophenone Derivatives
from Various Vehicles,” International Journal of Cosmetic Science, vol. 23(4), pp. 211-218,
2001.
Leikauf, G.D., “Chapter 15: Toxic Responses of the Respiratory System,” C.D. Klaassen, editor.
Casarett and Doull's Toxicology: The Basic Science of Poisons, 8th ed., New York, NY:
McGraw-Hill; pp. 691-732, 2013.
Leite-Silva, V.R., M. Le Lamer, W.Y. Sanchez, et al., “The Effect of Formulation on the
Penetration of Coated and Uncoated Zinc Oxide Nanoparticles into the Viable Epidermis of
Human Skin in Vivo,” European Journal of Pharmaceutics and Biopharmaceutics, vol. 84(2),
pp. 297-308, 2013.
Li, C.H., C.C. Shen, Y.W. Cheng, et al., “Organ Biodistribution, Clearance, and Genotoxicity of
Orally Administered Zinc Oxide Nanoparticles in Mice,” Nanotoxicology, vol. 6(7), pp. 746-756,
2012.
Littleton, F., Jr., “Warfarin and Topical Salicylates,” Journal of the American Medical Society,
vol. 263(21), p. 2888, 1990.
Liu, X., D. Rua, A. Wokovich, et al., “Particle Size Distribution Analysis of OTC Aerosol or
Powder Drug Products with Potential for Inadvertent Inhalation Exposure to Consumers,”
Journal of Pharmaceutical Science, doi: 10.1016/j.xphs.2018.10.066 (Epub ahead of print),
2018, (available at https://www.ncbi.nlm.nih.gov/pubmed/30468827
, accessed May 25, 2021.
Liu, X., D. Rua, A. Wokovich, et al., “Particle Size Distribution Analysis of OTC Drug Products
with Unintended Inhalation Exposure to Consumers [abstract].” In: American Association of
Pharmaceutical Scientists Annual Meeting and Exposition; November 12-15, 2017; San Diego
Convention Center. Abstract number T1109, 2017.
Ma, R., B. Cotton, W. Lichtensteiger, et al., “UV Filters with Antagonistic Action at Androgen
Receptors in the MDA-kb2 Cell Transcriptional-Activation Assay,” Toxicological Sciences, vol.
74(1), pp. 43-50, 2003.
Mackie, B.S., and L.E. Mackie, “The PABA Story,” The Australasian Journal of Dermatology,
vol. 40(1), pp. 51-53, 1999.
Madan, R.K. and J. Levitt, “A Review of Toxicity from Topical Salicylic Acid Preparations,”
Journal of the American Academy of Dermatology, vol. 70(4), pp. 788-792, 2014.
Proposed Order OTC000008
Page 149
Marionnet, C., C. Pierrard, C. Golebiewski, et al., “Diversity of Biological Effects Induced by
Longwave UVA Rays (UVA1) in Reconstructed Skin,” PLoS One, vol. 9(8), 2014.
Matlack, S., “From Tanning Accessory to Health Necessity: History of the OTC Sunscreen
Monograph in Light of the Sunscreen Revolution,” 2009 (available at
https://nrs.harvard.edu/urn-3:HUL.InstRepos:8965570), accessed March 27, 2018.
Matta, M.K., J. Florian, R. Zusterzeel et al., “Effect of Sunscreen Application on Plasma
Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial,” Journal of the
American Medical Association, vol. 323(3), pp. 256-267, 2020 (available at
https://jamanetwork.com/journals/jama/fullarticle/2759002), accessed August 12, 2021.
Matta, M.K., R. Zusterzeel, R.P. Nageswara Matta et al., “Effect of Sunscreen Application
Under Maximal Use Conditions on Plasma Concentration of Sunscreen Active Ingredients: A
Randomized Clinical Trial,” Journal of the American Medical Association, vol. 321(21), pp.
2082-2091, 2019 (available at https://jamanetwork.com/journals/jama/fullarticle/2733085),
accessed August 12, 2021.
McKinlay, A.F. and B.L. Diffey, “A Reference Action Spectrum for Ultraviolet Induced
Erythema in Ruman Skin,” CIE-Journal, vol. 6, pp. 17-22, 1987.
Mitchnick, M.A., D. Fairhurst, and S.R. Pinnell, “Microfine Zinc Oxide (Z-Cote) as a
Photostable UVA/UVB Sunblock Agent,” Journal of the American Academy of Dermatology,
vol. 40(1), pp. 85-90, 1999.
Monteiro-Riviere, N.A., K. Wiench, R. Landsiedel, et al., “Safety Evaluation of Sunscreen
Formulations Containing Titanium Dioxide and Zinc Oxide Nanoparticles in UVB Sunburned
Skin: An in Vitro and in Vivo Study,” Toxicological Sciences, vol. 123(1), pp. 264-280, 2011.
Montenegro, L. and G. Puglisi, “Evaluation of Sunscreen Safety by in Vitro Skin Permeation
Studies: Effects of Vehicle Composition,” Die Pharmazie, vol. 68(1), pp. 34-40, 2013.
Montenegro, L., C. Carbone, D. Paolino, et al., “In Vitro Skin Permeation of Sunscreen Agents
from O/W Emulsions,” International Journal of Cosmetic Science, vol. 30(1), pp. 57-65, 2008.
Montemarano, A., R. Gupta, J. Burge, et al., “Insect Repellents and the Efficacy of Sunscreens,”
The Lancet, vol. 349(9066), pp. 1670-1671, 1997.
Mouret, S., C. Baudouin, M. Charveron, et al., “Cyclobutane Pyrimidine Dimers Are
Predominant DNA Lesions in Whole Human Skin Exposed to UVA Radiation,” Proceedings of
the National Academies of Sciences of the United States of America, vol. 103(37), pp. 13765-
13770, 2006.
Nakamura, N., A.L. Inselman, G.A. White, et al., “Effects of Maternal and Lactational Exposure
to 2-Hydroxy-4-Methoxybenzone on Development and Reproductive Organs in Male and

Proposed Order OTC000008
Page 150
Female Rat Offspring,” Birth Defects Research. Part B, Developmental and Reproductive
Toxicology, vol. 104(1), pp. 35-51, 2015.
Nallagundla, S., S. Patnala, and I. Kanfer, “Comparison of In Vitro Release Rates of Acyclovir
from Cream Formulations Using Vertical Diffusion Cells,” AAPS PharmSciTech, vol. 15(4), pp.
994-999, 2014.
Nash, J.F., and P.R. Tanner, “Relevance of UV Filter/Sunscreen Product Photostability to
Human Safety,” Photodermatology, Photoimmunology and Photomedicine, vol. 30(2-3), pp. 88-
95, 2014.
*National Cancer Institute, “Cancer Trends Progress Report,” February 2018 (available at
https://progressreport.cancer.gov), accessed May 25, 2021.
*National Science and Technology Council Committee on Technology, “The National
Nanotechnology Initiative Strategic Plan,” February 2014 (available at
http://www.nano.gov/sites/default/files/pub_resource/2014_nni_strategic_plan.pdf), accessed
May 25, 20221.
*National Toxicology Program, “Nomination Summary for 2-Hydroxy-4-methoxybenzophenone
(N79456),” 1979 (available at https://ntp.niehs.nih.gov/testing/noms/search/summary/nm-
n79456.html), accessed May 26, 2021.
*National Toxicology Program, NTP Technical Report 518, “Toxicology and Carcinogenesis
Studies of Triethanolamine in B6C3F
1
Mice (Dermal Study),” May 2004 (available at
https://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr518.pdf
), accessed May 26, 2021.
*National Toxicology Program, Technical Report 449, “Toxicology and Carcinogenesis Studies
of Triethanolamine in F344/N Rats and B6C3F
1
Mice (Dermal Studies),” November 1999,
(available at https://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr449.pdf
), accessed May 26, 2021.
*National Toxicology Program, “Testing Status of 2-Hydroxy-4-methoxybenzophenone 10260-
S” (available at https://ntp.niehs.nih.gov/testing/status/agents/ts-10260-s.html), accessed May 26,
2021.
Ng, S.F., J.J. Rouse, F.D. Sanderson, et al., “Validation of a Static Franz Diffusion Cell System
for In Vitro Permeation Studies,” AAPS PharmSciTech, vol. 11(3), pp. 1432-1441, 2010.
Noonan, F.P., M.R. Zaidi, A. Wolnicka-Glubisz, et al., “Melanoma Induction by Ultraviolet A
but Not Ultraviolet B Radiation Requires Melanin Pigment,” Nature Communications, vol. 3, pp.
884, 2012.
Palm, M.D. and M.N. O’Donoghue, “Update on Photoprotection,” Dermatologic Therapy, vol.
25(5), pp. 360-376, 2007.
Proposed Order OTC000008
Page 151
Pastore, M.N., Y.N. Kalia, M. Horstmann, et al., “Transdermal Patches: History, Development
and Pharmacology,” British Journal of Pharmacology, vol. 172(9), pp. 2179-2209, 2015.
Pathak, M.A., “Sunscreens: Topical and Systemic Approaches for Protection of Human Skin
against Harmful Effects of Solar Radiation,” Journal of the American Academy of Dermatology,
vol. 7(3), pp. 285-312, 1982.
Pflucker, F., V. Wendel, H. Hohenberg, et al., “The Human Stratum Corneum Layer: An
Effective Barrier against Dermal Uptake of Different Forms of Topically Applied Micronised
Titanium Dioxide,” Skin Pharmacology and Applied Skin Physiology, vol. 14 Suppl 1, pp. 92-97,
2001.
Plum, L.M., L. Rink, and H. Haase, “The Essential Toxin: Impact of Zinc on Human Health,”
International Journal of Environmental Research and Public Health, vol. 7(4), pp. 1342-1365,
2010.
Potard, G., C. Laugel, H. Schaefer, et al., “The Stripping Technique: In Vitro Absorption and
Penetration of Five UV Filters on Excised Fresh Human Skin,” Skin Pharmacology and Applied
Skin Physiology, vol. 13(6), pp. 336-344, 2000.
Premi, S., S. Wallisch, C.M. Mano, et al., “Photochemistry. Chemiexcitation of Melanin
Derivatives Induces DNA Photoproducts Long after UV Exposure,” Science, vol. 347(6224), pp.
842-847, 2015.
Raney, S., P. Lehman, and T. Franz, “30th Anniversary of the Franz Cell Finite Dose Model:
The Crystal Ball of Topical Drug Development,” Journal of Drug Delivery Science and
Technology, vol. 8(7), pp. 32-37, 2008.
Ridley, A.J., J.R. Whiteside, T.J. McMillan, et al., “Cellular and Sub-Cellular Responses to UVA
in Relation to Carcinogenesis,” International Journal of Radiation Biology, vol. 85(3), pp. 177-
195, 2009.
Rodriguez, E., M.C. Valbuena, M. Rey, et al., “Causal Agents of Photoallergic Contact
Dermatitis Diagnosed in the National Institute of Dermatology of Colombia,” Photodermatology,
Photoimmunology and Photomedicine, vol. 22(4), pp. 189-192, 2006.
Rose, F.A. and D.R. Wiemer, “Platelet Coagulopathy Secondary to Topical Salicylate Use,”
Annals of Plastic Surgery, vol. 11(4), pp. 340-343, 1983.
Ross, E.A., K.A. Savage, L.J. Utley, et al., “Insect Repellant Interactions: Sunscreens Enhance
DEET (N,N-Diethyl-M-Toluamide) Absorption,” Drug Metabolism and Disposition, vol. 32,
pp.783-785, 2004.
Rothe, H., R. Fautz, E. Gerber, et al., “Special Aspects of Cosmetic Spray Safety Evaluations:
Principles on Inhalation Risk Assessment,” Toxicology Letters, vol. 205(2), pp. 97-104, 2011.
Proposed Order OTC000008
Page 152
Ryu, H.J., M.Y. Seo, S.K. Jung, et al., “Zinc Oxide Nanoparticles: A 90-Day Repeated-Dose
Dermal Toxicity Study in Rats,” International Journal of Nanomedicine, vol. 9 Suppl 2, pp. 137-
144, 2014.
Sadrieh, N., A.M. Wokovich, N.V. Gopee, et al., “Lack of Significant Dermal Penetration of
Titanium Dioxide from Sunscreen Formulations Containing Nano- and Submicron-Size Tio2
Particles,” Toxicological Sciences, vol. 115(1), pp. 156-166, 2010.
Samman, S. “Zinc.” J.I. Mann and A.S. Truswell, editors, Essentials of Human Nutrition, 3rd
ed., Oxford: Oxford University Press, pp. 138-142, 2007.
Sandstead, H.H., “Zinc.” B.A. Fowler and M. Nordberg, editors, Handbook on the Toxicology of
Metals (Fourth Edition), San Diego: Academic Press, 2015: 1369-1385.
Santos, P., A.C. Watkinson, J. Hadgraft, et al., “Influence of Penetration Enhancer on Drug
Permeation from Volatile Formulations,” International Journal of Pharmaceuticals, vol. 439, pp.
260-268, 2012.
Sarveiya, V., S. Risk, and H. Benson, “Liquid Chromatographic Assay for Common Sunscreen
Agents: Application to In Vivo Assessment of Skin Penetration and Systemic Absorption in
Human Volunteers,” Journal of Chromatography, vol. 803(2), pp. 225-231, 2004.
Schauder, S. and H. Ippen, “Contact and Photocontact Sensitivity to Sunscreens. Review of a 15-
Year Experience and of the Literature,” Contact Dermatitis, vol. 37(5), pp. 221-232, 1997.
Schiffer, C., A. Muller, D.L. Egeberg, et al., “Direct Action of Endocrine Disrupting Chemicals
on Human Sperm,” European Molecular Biology Organization Reports, vol. 15(7), pp. 758-765,
2014.
Schlumpf, M., P. Schmid, S. Durrer, et al., “Endocrine Activity and Developmental Toxicity of
Cosmetic UV Filters--an Update,” Toxicology, vol. 205(1-2), pp. 113-122, 2004.
88. Schlumpf, M., B. Cotton, M. Conscience, et al., “In Vitro and in Vivo Estrogenicity of UV
Screens,” Environmental Health Perspectives, vol. 109(3), pp. 239-244, 2001.
Schreurs, R., P. Lanser, W. Seinen, et al., “Estrogenic Activity of UV Filters Determined by an
in Vitro Reporter Gene Assay and an in Vivo Transgenic Zebrafish Assay,” Archives of
Toxicology, vol. 76(5-6), pp. 257-261, 2002.
Schreurs, R.H., E. Sonneveld, J.H. Jansen, et al., “Interaction of Polycyclic Musks and UV
Filters with the Estrogen Receptor (Er), Androgen Receptor (Ar), and Progesterone Receptor (Pr)
in Reporter Gene Bioassays,” Toxicological Sciences, vol. 83(2), pp. 264-272, 2005.
Schulz, J., H. Hohenberg, F. Pflucker, et al., “Distribution of Sunscreens on Skin,” Advanced
Drug Delivery Reviews, vol. 54 Suppl 1, pp. S157-163, 2002.

Proposed Order OTC000008
Page 153
Seidlova-Wuttke, D., H. Jarry, J. Christoffel, et al., “Comparison of Effects of Estradiol (E2)
with Those of Octylmethoxycinnamate (OMC) and 4-Methylbenzylidene Camphor (4MBC)--2
Filters of UV Light--on Several Uterine, Vaginal and Bone Parameters,” Toxicology and Applied
Pharmacology, vol. 210(3), pp. 246-254, 2006.
Simeoni, S., S. Scalia, and H.A. Benson, “Influence of Cyclodextrins on in Vitro Human Skin
Absorption of the Sunscreen, Butyl-Methoxydibenzoylmethane,” International Journal of
Pharmaceutics, vol. 280(1-2), pp. 163-171, 2004.
Stanfield, J.W., H. Ou-Yang, T. Chen, et al., “Multi-Laboratory Validation of Very High Sun
Protection Factor Values,” Photodermatology, Photoimmunology & Photomedicine, vol. 27(1),
pp. 30-34, 2011.
Stefanidou, M., C. Maravelias, A. Dona, et al., “Zinc: A Multipurpose Trace Element,” Archives
of Toxicology, vol. 80(1), pp. 1-9, 2006.
Steiling W., M. Bascompta, P. Carthew, et al., “Principle Considerations for the Risk
Assessment of Sprayed Consumer Products,” Toxicology Letters, vol. 227(1), pp. 41-49, 2014.
Stenberg, C., and O. Larko, “Sunscreen Application and Its Importance for the Sun Protection
Factor,” Archives of Dermatology, vol. 121(11), pp. 1400-1402, 1985.
Stuart, B.O., “Deposition and Clearance of Inhaled Particles,” Environmental Health
Perspectives, vol. 55, pp. 369-390, 1984.
Svarc, F., “A Brief Illustrated History on Sunscreens and Sun Protection,” Pure and Applied
Chemistry, vol. 87(9-10), pp. 929-936, 2015.
Swedish Research Council, “Sunscreens with Benzophenone-3 Unsuitable for Children,” Public
release date: November 6, 2006 (available at
https://www.eurekalert.org/pub_releases/2006-
11/src-swb110606.php), accessed May 26, 2021.
Szwarcfarb, B., S. Carbone, R. Reynoso, et al., “Octyl-Methoxycinnamate (OMC), an Ultraviolet
(UV) Filter, Alters LHRH and Amino Acid Neurotransmitters Release from Hypothalamus of
Immature Rats,” Experimental and Clinical Endocrinology and Diabetes, vol. 116(2), pp. 94-98,
2008.
Teplitz, R.W., A.M. Glazer, R.M. Svoboda, et al., “Trends in US Sunscreen Formulations:
Impact of Increasing Spray Usage,” Journal of the American Academy of Dermatology, vol.
78(1), pp. 187-189, 2018.
Tewari, A., R.P. Sarkany, and A.R. Young, “UVA1 Induces Cyclobutane Pyrimidine Dimers but
Not 6-4 Photoproducts in Human Skin in Vivo,” Journal of Investigative Dermatology, vol.
132(2), pp. 394-400, 2012.

Proposed Order OTC000008
Page 154
Tewari, A., M.M. Grage, G.I. Harrison, et al., “UVA1 Is Skin Deep: Molecular and Clinical
Implications,” Photochemical and Photobiological Sciences, vol. 12(1), pp. 95-103, 2013.
*Therapeutic Goods Administration, “Australian Regulatory Guidelines for Sunscreens
(ARGS),” TGA Publication, pp. 1-43, 2016.
Treffel, P. and B. Gabard, “Skin Penetration and Sun Protection Factor of Ultra-Violet Filters
from Two Vehicles,” Pharmaceutical Research, vol. 13(5), pp. 770-774, 1996.
Trevisi, P., C. Vincenzi, C. Chieregato, et al., “Sunscreen Sensitization: A Three-Year Study,”
Dermatology (Basel, Switzerland), vol. 189(1), pp. 55-57, 1994.
Unnithan, J., M.U. Rehman, F.J. Ahmad, et al., “Aqueous Synthesis and Concentration-
Dependent Dermal Toxicity of Tio2 Nanoparticles in Wistar Rats,” Biological Trace Element
Research, vol. 143(3), pp. 1682-1694, 2011a.
Unnithan, J., M.U. Rehman, F.J. Ahmad, et al., “Concentration Dependent Toxicity of
Approximately 20 Nm Anatase Titanium Dioxide Nanoparticles--an in Vivo Study on Wistar
Rats,” Journal of Biomedical Nanotechnology, vol. 7(1), pp. 207-208, 2011b.
Urbach, F., “The Historical Aspects of Sunscreens,” Journal of Photochemistry and
Photobiology B, vol. 64(2-3), pp. 99-104, 2001.
*U.S. Department of Health and Human Services, “The Surgeon General’s Call to Action to
Prevent Skin Cancer,” (available at
https://www.hhs.gov/surgeongeneral/reports-and-
publications/skin-cancer/executive-summary/index.html), accessed May 26, 2021.
U.S. Pharmacopeia, “<429> Light Diffraction Measurement of Particle Size,” US Pharmacopeia
43--National Formulary 38, pp. 1-5, June 2021.
U.S. Pharmacopeia, “1724: Semisolid Drug Products--Performance Tests,” US Pharmacopeia
40--National Formulary 35, pp. 2055-2067, December 2017.
Ulrich, C., J.S. Jurgensen, A. Degen, et al., “Prevention of Non-Melanoma Skin Cancer in Organ
Transplant Patients by Regular Use of a Sunscreen: a 24 Months, Prospective, Case-Control
Study,” The British Journal of Dermatology, vol. 161 Suppl 3, pp. 78-84, 2009.
Valbuena Mesa, M.C. and E.V. Hoyos Jimenez, “Photopatch Testing in Bogota (Colombia):
2011-2013,” Contact Dermatitis, vol. 74(1), pp. 11-17, 2016.
Victor, F.C., D.E. Cohen, and N.A. Soter, “A 20-Year Analysis of Previous and Emerging
Allergens That Elicit Photoallergic Contact Dermatitis,” Journal of the American Academy of
Dermatology, vol. 62(4), pp. 605-610, 2010.
Proposed Order OTC000008
Page 155
Walters, K.A., A.C. Watkinson, and K.R. Brain, “In Vitro Skin Permeation Evaluation: The
Only Realistic Option,” International Journal of Cosmetic Science, vol. 20(5), pp. 307-316,
1998.
Walters, K.A., K.R. Brain, D. Howes, et al., “Percutaneous Penetration of Octyl Salicylate from
Representative Sunscreen Formulations through Human Skin in Vitro,” Food and Chemical
Toxicology, vol. 35(12), pp. 1219-1225, 1997.
Wang, T. and X. Gu, “In Vitro Percutaneous Permeation of the Repellent DEET and the
Sunscreen Oxybenzone Across Human Skin,” Journal of Pharmacy and Pharmaceutical
Sciences, vol. 10(1), pp. 17-25, 2007.
Wang, S.Q., J.W. Stanfield, and U. Osterwalder, “In Vitro Assessments of UVA Protection by
Popular Sunscreens Available in the United States,” Journal of the American Academy of
Dermatology, vol. 59(6), pp. 934-942, 2008.
Wang, L.H., W.S. Huang, and H.M. Tai, “Simultaneous Determination of P-Aminobenzoic Acid
and its Metabolites in the Urine of Volunteers, Treated with P-Aminobenzoic Acid Sunscreen
Formulation,” Journal of Pharmaceutical and Biomedical Analysis, vol. 43(4), pp. 1430-1436,
2007.
Warheit, D.B., R. Boatman, and S.C. Brown, “Developmental Toxicity Studies with 6 Forms of
Titanium Dioxide Test Materials (3 Pigment-Different Grade & 3 Nanoscale) Demonstrate an
Absence of Effects in Orally-Exposed Rats,” Regulatory Toxicology and Pharmacology, vol.
73(3), pp. 887-896, 2015.
Warshaw, E.M., M.Z. Wang, H.I. Maibach, et al., “Patch Test Reactions Associated with
Sunscreen Products and the Importance of Testing to an Expanded Series: Retrospective
Analysis of North American Contact Dermatitis Group Data, 2001 to 2010,” Dermatitis, vol.
24(4), pp. 176-182, 2013.
Willis, I., “Sensitization Potential of Para-Aminobenzoic Acid,” Cosmetics and Toiletries, vol.
91(3), pp. 63-64, 1976.
Xu, S., M. Kwa, A. Agarwal, et al. “Sunscreen Product Performance and Other Determinants of
Consumer Preferences,” Journal of the American Medical Association Dermatology, vol. 52(8),
pp. 920-927, 2016.
Yiin, L. M., J.N. Tian, and C.C. Hung, “Assessment of Dermal Absorption of DEET-Containing
Insect Repellent and Oxybenzone-Containing Sunscreen Using Human Urinary Metabolites,”
Environmental Science and Pollution Research International, vol. 22, pp. 2062-2070, 2015.
Ziegler, A., A.S. Jonason, D.J. Leffell, et al., “Sunburn and P53 in the Onset of Skin-Cancer,”
Nature, vol. 372(6508), pp. 773-776, 1994.
Proposed Order OTC000008
Page 156
Theresa M. Michele, MD
Director, Office of Nonprescription Drugs
Center for Drug Evaluation and Research
September 24, 2021
Theresa M.
Michele -S
Digitally signed by Theresa M. Michele -S
DN: c=US, o=U.S. Government, ou=HHS,
ou=FDA, ou=People,
0.9.2342.19200300.100.1.1=1300428996,
cn=Theresa M. Michele -S
Date: 2021.09.24 06:55:36 -04'00'
