
PROFESSIONAL PRODUCT INFORMATION
Guidelines
for the Management
of Acetaminophen Overdose

(acetaminophen)
38
This brochure outlines basic steps in the management of
acetaminophen overdose and reviews the application of
these management principles to special populations. It is
a revision of previous publications and should be used in
place of earlier versions. Included herein are flowcharts
for managing both acute and chronic acetaminophen
overdose, and a nomogram, which uses acetaminophen
serum concentrations at various time intervals following a
single, acute overdose to determine whether the antidote
should be administered.
In January 1985, the United States (US) Food and Drug Administration
(FDA) approved the oral administration of acetylcysteine (N-acetylcys-
teine, NAC) as an antidote for the treatment of acetaminophen overdose.
Approval of acetylcysteine for this purpose was based on a nationwide
research program conducted by the Rocky Mountain Poison and Drug
Center under the sponsorship of McNeil Consumer Healthcare. This
research clearly demonstrated the efficacy of acetylcysteine, when used
early in the course of treatment, in reducing morbidity and virtually elim-
inating mortality associated with acetaminophen overdose. In 2004, the
FDA approved the intravenous formulation of acetylcysteine (Acetadote
®
,
Cumberland Pharmaceuticals, Nashville, TN).
(482)
This monograph is intended to assist practitioners in managing acet-
aminophen overdoses and is not meant as a standard of care. For further
information concerning complex or difficult cases, please contact your
local poison center (1-800-222-1222) or a clinical toxicologist. McNeil Con-
sumer Healthcare sponsors a toll free telephone number (1-800-525-6115),
available 24 hours a day, at the Rocky Mountain Poison and Drug Center.
Please do not hesitate to use these resources if you need individualized
consultation on managing a patient with an acetaminophen overdose.
If you would like additional information about TYLENOL
®
(acetamino-
phen), or additional copies of this management protocol, please contact
us at the address below.
McNeil Consumer Healthcare
7050 Camp Hill Road
Fort Washington, PA 19034
Prepared by the consultant panel:
G Randall Bond, MD
E. Martin Caravati, MD, MPH
Richard C. Dart, MD, PhD
Kennon Heard, MD
Robert S. Hoffman, MD
Barry H. Rumack, MD
Wayne R. Snodgrass, MD, PhD
With support from McNeil Consumer Healthcare Division of McNEIL-PPC, Inc.
Guidelines
for the Management
of Acetaminophen Overdose

(acetaminophen)
39
Guidelines for the Management
of Acetaminophen Overdose 38
Table of Contents 39
Introduction 40
Definitions 41
Management of Acute Overdose 42
1. Initial Assessment 42
2. Gastric Decontamination/Prevention of Absorption 42
3. Determining the Need for Acetylcysteine 43
Acetaminophen Assay ...............................43
4. Administration of Acetylcysteine 43
a. Choose a route of administration ...................43
b. Transitioning from oral to intravenous
acetylcysteine treatment. . . . . . . . . . . . . . . . . . . . . . . . . . . 44
c. Continuation of acetylcysteine treatment ...........44
5. Other Laboratory Tests 44
6. Supportive Treatment 44
7. Special Considerations 45
a. Extended release acetaminophen ...................45
b. Ingestion of acetaminophen combination products . . 45
c. Massive acetaminophen ingestion ..................45
d. Intravenous acetaminophen ........................45
8. Special Populations 46
a. Young children (<6 years of age) ....................46
b. Pregnant women ..................................46
c. Patients presenting 24 hours or more postingestion ..46
d. Chronic alcohol users ..............................46
e. Obese patients ....................................46
f. Other diseases .....................................46
Management of Repeated Chronic
Supratherapeutic Ingestion 47
Clinical Characteristics
of Acute Acetaminophen Overdose 48
Phase I 48
Phase II 48
Phase III 48
Summary 49
Acetaminophen Overdose:
Suggested Readings 54
Table of Contents
List of Figures, Flowcharts, and Charts
Flowchart 1. Stepwise Management
of Acute Acetaminophen Overdose 50
Flowchart 2. Stepwise Management
of Repeated Supratherapeutic Ingestion 51
Chart 1. Rumack-Matthew Nomogram 52
Chart 2. Common Adverse Events Associated
with the Oral and Intravenous Formulations
of n-acetylcysteine. 53

(acetaminophen)
40
Introduction
An overdose of acetaminophen may result in severe liver
injury. Acetylcysteine is an effective antidote to prevent
or limit liver injury in patients with potentially toxic
acetaminophen levels or evidence of liver injury.

(acetaminophen)
41
Definitions
Overdosage of acetaminophen can occur following an
acute overdose or during repeated overdose. Acute
acetaminophen overdose is defined as an ingestion
of a toxic amount of acetaminophen occurring within
a period of 8 hours or less. In adults and adolescents,
hepatotoxicity may occur following ingestion of greater
than 7.5 to 10 grams (g) (eg, 24 regular-strength or 15
extra-strength caplets or tablets) over a period of 8 hours
or less. Fatalities are infrequent especially when treated
with acetylcysteine (0.3% of treated cases).
A chronic overdose is termed repeated supratherapeutic
ingestion (RSTI) to differentiate from chronic therapeutic
use. Ingestion of a toxic amount over a period greater
than 8 hours is considered a repeated supratherapeutic
ingestion.
Acetylcysteine is the official term designated by USAN
(United States Adopted Names) for N-acetylcysteine.

(acetaminophen)
42
Management
of Acute Overdose
To achieve optimal outcome following acetaminophen
overdose, a systematic management approach is
needed. This section outlines basic steps in managing
acute acetaminophen overdose, consistent with FDA
approved labeling of acetylcysteine. Flowchart 1
outlines this stepwise approach.
1. Initial Assessment
Adults or adolescents (*12 years of age) who may have ingested
acetaminophen in a purposeful overdose, independent of the amount
reported to have been ingested, should be referred for medical evalu-
ation. Their evaluation includes careful estimation of the quantity and
dosage form of the acetaminophen ingested as well as assessment of any
other substances ingested. The acetaminophen level should be deter-
mined at 4 hours post ingestion or as soon as possible thereafter (see
also Special Considerations).
Patients who present with a measurable acetaminophen level and no
clear time of exposure represent a treatment challenge and there is sub-
stantial practice variation. In some cases it is possible to develop a “worst
case scenario” for the time of ingestion (e.g. the patient was with their
family until 12 hours prior to presentation so ingestion could not have
occurred more than 12 hours prior). In these cases, the earliest possible
time of ingestion should be used to plot the acetaminophen level on the
nomogram (Chart 1). If the time of ingestion is completely unknown, the
most conservative approach is to initiate treatment and continue acetyl-
cysteine until the acetaminophen level is undetectable and there is no
evidence of progressive hepatic injury (serum transaminases normal or
near normal and stable over a 12 hour period).
There is substantial practice variation in the management of patients who
have low level transaminase elevations and a questionable history of the
time and amount of acetaminophen exposure. In many of these cases the
acetaminophen level may be therapeutic or even undetectable. The most
conservative approach in these cases is to initiate treatment and continue
acetylcysteine until the acetaminophen level is undetectable and there is
no evidence of progressive hepatic injury (serum transaminases normal
or near normal and stable over a 12 hour period).
2. Gastric Decontamination/Prevention of Absorption
Gastric decontamination should be carried out according to standard
treatment guidelines. Activated charcoal reduces the peak serum con-
centration of acetaminophen. This may reduce the 4 hour acetaminophen
level and thereby decrease the number of patients requiring treatment
with acetylcysteine. Activated charcoal may be given during the immedi-
ate postingestion period, especially in the case of a mixed drug overdose.
Data supporting the efficacy of activated charcoal beyond 2 hours after
ingestion are limited. Administration of activated charcoal does not
require a change in subsequent administration of oral or intravenous
acetylcysteine therapy.

(acetaminophen)
43
3. Determining the Need for Acetylcysteine
Acetaminophen Assay
Rationale
The acetaminophen level provides the basis for determining the need to
initiate or continue treatment with acetylcysteine. Either the plasma or
serum acetaminophen level may be used; most hospitals determine the
serum acetaminophen level. The serum acetaminophen level should be
measured at 4 hours following ingestion of an acute overdose or as soon
as possible thereafter. It is important to determine the time of ingestion
accurately. If the ingestion occurred over a period of time, the time of the
initial ingestion is used for plotting on the Rumack-Matthew nomogram
(Chart 1). (For example, if the ingestion occurred over the period of 6 PM
to 8 PM, the acetaminophen level could be drawn at 10 PM and would be
plotted as a 4 hour level on the nomogram.)
When to obtain
Blood should be drawn immediately for the acetaminophen serum assay
if 4 hours or more have elapsed postingestion. If less than 4 hours have
elapsed postingestion, it is important to wait until the 4 hour point to
draw blood. If the acetaminophen level is clearly in the toxic range (ie,
above the treatment line on the Rumack-Matthew nomogram), dosing
with acetylcysteine should be initiated immediately. Use of levels
obtained before 4 hours has not been studied and may not be reliable.
Such levels should not be plotted on the nomogram (Chart 1).
If an assay for acetaminophen cannot be obtained, it is necessary to
assume that the overdose is potentially toxic and acetylcysteine treat-
ment should be initiated. Treatment should continue for the full course
of therapy or until an acetaminophen level can be obtained and is clearly
below the treatment line on the treatment nomogram (Chart 1).
Interpretation of acetaminophen assays
The Rumack-Matthew nomogram is used to interpret the acetaminophen
level (Chart 1). If the initial acetaminophen level plots above the treatment
line (starting at 150 mcg/mL at 4 hours), then acetylcysteine treatment
is recommended. If the initial acetaminophen level, determined at least
4 hours following an overdose, plots below the treatment line described
above, the risk of hepatotoxicity is minimal and acetylcysteine treatment
is not necessary. If already initiated, the acetylcysteine treatment can be
discontinued. (see also Special Considerations: Ingestion of acetamino-
phen combination products)
Only the initial acetaminophen level is used in making the decision to initi-
ate or continue acetylcysteine treatment (see also Special Considerations:
Extended-Release Acetaminophen and Ingestion of Acetaminophen
Combination Products). A complete course of acetylcysteine should be
provided if the initial level is above the treatment line, even if subsequent
acetaminophen levels plot below the treatment line.
4. Administration of Acetylcysteine
If a patient presents within 4 hours of an acute overdose, treatment with
acetylcysteine should be withheld until acetaminophen assay results are
available, provided that initiation of treatment is not delayed beyond 8
hours following the ingestion.
If a patient with a potential acetaminophen overdose presents for care
more than 8 hours after ingestion, acetylcysteine should be administered
immediately, regardless of the quantity of acetaminophen reported to
have been ingested. It is important not to wait for results of the acetamin-
ophen assay before initiating acetylcysteine.
There are multiple treatment protocols for managing acetaminophen
overdoses. While there are substantial variations in treatment practices,
we are presenting 2 commonly accepted treatment protocols.
The following procedures are recommended:
a. Choose a route of administration
Both intravenous and oral formulations of acetylcysteine are available
and approved by the US FDA. The oral formulation has been used for many
years in the United States. Intravenous administration has become the most
common route of acetylcysteine treatment (www.acetadote.net); however,
either the oral or intravenous drugs are acceptable for most patients.
The primary adverse events of concern with the intravenous formulation of
acetylcysteine are anaphylactoid reactions such as pruritus and broncho-
spasm. In rare cases death has occurred. The primary adverse events with
the oral formulation are nausea and vomiting which can lead to insufficient
absorption of the administered dose. See Chart 2 for a list of common
adverse events associated with the intravenous and oral formulations.
i) Intravenous administration
The US FDA approved regimen for the intravenous administration of ace-
tylcysteine (Acetadote
®
) involves 3 sequential infusions over a total period
of 21 hours. For patients with body weight above 40 kg, the loading dose
is 150 mg/kg in 200 mL of 5% dextrose (D5W), infused over 60 minutes.
The second infusion is 50 mg/kg in 500 mL D5W, infused over 4 hours
(12.5 mg/kg/h). The third infusion is 100 mg/kg in 1000 mL D5W infused
over 16 hours (6.25 mg/kg/h). In patients weighing less than 40kg, this
dosing regimen provides too much free water and can cause hyponatre-
mia and seizures. The package insert should be referenced when treating
patients weighing less than 40kg.
ii) Oral administration
The US FDA approved dosage regimen of oral acetylcysteine involves a load-
ing dose of 140 mg/kg followed by 17 doses of 70 mg/kg at 4 hour intervals
(total duration of treatment, 72 hours). If the patient vomits the loading dose
or any maintenance dose within 1 hour of administration, the patient should
be switched to the intravenous formulation (see product prescribing informa-
tion for complete details). Some toxicologists have adopted shorter courses
of oral therapy based on their own specific clinical parameters*.
MANAGEMENT OF ACUTE OVERDOSE
* This monograph is intended to assist practitioners in managing acetaminophen overdoses and is not meant as a standard of care. For further information
and individualized consultation concerning complex or difficult cases, please contact your local poison center (1-800-222-1222) or a clinical toxicologist.
McNeil Consumer Healthcare sponsors a toll-free telephone number (1-800-525-6115), available 24 hours a day, at the Rocky Mountain Poison and Drug Center.

(acetaminophen)
44
b. Transitioning from oral to intravenous acetylcysteine treatment
If a clinician determines that a patient who has received oral acetylcyste-
ine should be transitioned to intravenous acetylcysteine we recommend
the following approach:
i) If the patient has vomited a loading dose of oral acetylcysteine within 60
minutes, begin with the first infusion of the intravenous protocol.
ii) If the patient has received only a loading dose (140 mg/kg) of oral
acetylcysteine and retained it for more than 60 minutes, begin intra-
venous treatment with the second infusion of the intravenous protocol
(12.5 mg/kg/hour for 4 hours).
iii) If the patient has received the oral loading dose (140 mg/kg) and any
subsequent oral doses (70 mg/kg), begin with the third infusion of
the intravenous protocol (6.25 mg/kg/hour).
c. Continuation of acetylcysteine treatment
Acetylcysteine should be continued beyond the standard time based
protocols for all patients with acetaminophen induced acute liver fail-
ure. Acute liver failure is defined by a rapid decline in hepatic function
characterized by jaundice, coagulopathy (international normalized ratio
(INR) >1.5), and hepatic encephalopathy in patients with no evidence of
prior liver disease. Treatment may be stopped when the patient’s hepatic
encephalopathy has resolved and their clinical condition is improving.
The exact duration of treatment may vary.
i) Intravenous administration
In most cases, it is recommended that intravenous acetylcysteine treat-
ment be continued until the patient is clearly improving or transplant is
performed. A reasonable endpoint is an alanine transaminase (ALT) of
<50% of peak values, an international normalized ratio (INR) <2.0* and an
acetaminophen level <10 mcg/mL.
ii) Oral administration
In most cases, prolonged administration of acetylcysteine will utilize the
intravenous route of administration. If administration of the oral solution
is continued, a common approach is to continue the maintenance dose
every 4 hours (eg, 70 mg/kg every 4 hours) until the patient is clearly
improving or transplant is performed. A reasonable endpoint is an ALT of
<50% of peak values, an INR <2.0* and an acetaminophen level <10 mcg/mL.
5. Other Laboratory Tests
i)
In healthy, asymptomatic patients who present early after acute acet-
aminophen ingestion, only an acetaminophen level is needed.
ii) In a patient with an acetaminophen level above the nomogram treat-
ment line, an ALT and aspartate transaminase (AST) level should be
obtained. The ALT or AST should be determined at the end of ace-
tylcysteine infusion (18-20 hours) and repeated every 12 to 24 hours
until the patient recovers. If the ALT or AST remains elevated or the
acetaminophen level is >10 mcg/mL, the acetylcysteine infusion should
be continued until the patient is clearly improving or transplant is per-
formed. A reasonable endpoint is an ALT <50% of peak values an INR
<2.0* and an acetaminophen level <10 mcg/mL.
iii) A number of abnormal laboratory tests (prothrombin time (PT) or
INR, bilirubin, phosphate, lactate and pH) are associated with a poor
prognosis and should be assessed serially in patients with severe liver
injury. When such abnormalities are present, consultation may be
indicated*.
6. Supportive Treatment
i)
It is important to monitor for signs and symptoms of hepatic failure and
to provide appropriate supportive care.
ii) In cases in which fulminant hepatic failure develops, appropriate
toxicology and/or hepatology consultation should be obtained. In rare
cases, referral to a transplant center may be necessary.
MANAGEMENT OF ACUTE OVERDOSE
* This monograph is intended to assist practitioners in managing acetaminophen overdoses and is not meant as a standard of care. For further information
and individualized consultation concerning complex or difficult cases, please contact your local poison center (1-800-222-1222) or a clinical toxicologist.
McNeil Consumer Healthcare sponsors a toll-free telephone number (1-800-525-6115), available 24 hours a day, at the Rocky Mountain Poison and Drug Center.

(acetaminophen)
45
7. Special Considerations
a. Extended release acetaminophen
There are multiple products available that contain an extended release
formulation of acetaminophen. In cases of overdose, the concern is that
absorption of extended release acetaminophen is slower than that of
immediate release acetaminophen. As a result, the acetaminophen level
could plot below the treatment line of the nomogram at 4 hours, but rise
above the treatment line with continued absorption.
i) After an acute overdose with an extended release acetaminophen
product, the acetaminophen level should be measured at 4 hours after
ingestion or as soon as possible thereafter. Because of differences in
absorption rates, the significance of delayed rising levels is not clear.
Some toxicologists recommend obtaining a second acetaminophen
level 4 to 6 hours after the first measurement, whereas others do not.
Until there is further evidence, it may be prudent to obtain a second level.
ii) If either of the acetaminophen levels plot above the treatment line
of the nomogram, a full course of acetylcysteine treatment should
be administered.
iii) If both levels plot below the treatment line, toxicity is unlikely and
acetylcysteine treatment is not necessary and, if already initiated,
can be discontinued.
b. Ingestion of acetaminophen combination products
The ingestion of acetaminophen-diphenhydramine or acetamino-
phen-opioid products have been associated with delayed elevations
of the acetaminophen level. Patients with rising acetaminophen levels
require closer management and may require prolongation of acetylcys-
teine treatment*. For patients with initial acetaminophen levels that are
unexpectedly low, or with exposures involving the above combination
products or additional drugs that could affect acetaminophen absorp-
tion, a second acetaminophen level at least 4 to 6 hours after the first
measurement is recommended.
c. Massive acetaminophen ingestion
While the clinical effects of acetaminophen ingestion are generally delayed
for many hours after the ingestion, there are reports of massive acetamino-
phen ingestion (greater than 50g) producing metabolic acidosis, lethargy,
coma and hyperglycemia within 4 hours post ingestion. Clinicians should
be aware of this unusual presentation of acetaminophen poisoning. Several
of these reports describe successful treatment of these patients using
standard acetylcysteine, but it may be prudent to treat longer than the
standard 21 hour protocol if the acetaminophen level remains detectable.
d. Intravenous acetaminophen
Intravenous acetaminophen is approved for the short term treatment of
mild to moderate pain and fever reduction in both adults and children in
numerous countries worldwide and was recently approved in the United
States. There have been several reports of young children experiencing
a 10-fold dosing error. The timing of risk assessment, indications for ace-
tylcysteine and optimal acetylcysteine dosing has not been established.
Consultation with your local toxicologist or poison center is recommended*.
MANAGEMENT OF ACUTE OVERDOSE
* This monograph is intended to assist practitioners in managing acetaminophen overdoses and is not meant as a standard of care. For further information
and individualized consultation concerning complex or difficult cases, please contact your local poison center (1-800-222-1222) or a clinical toxicologist.
McNeil Consumer Healthcare sponsors a toll-free telephone number (1-800-525-6115), available 24 hours a day, at the Rocky Mountain Poison and Drug Center.

(acetaminophen)
46
8. Special Populations
a. Young children (<6 years of age)
Serious toxicity and death have been extremely infrequent following an
acute acetaminophen overdose in young children, possibly because of
differences in acetaminophen metabolism. Oral overdoses in children
should be managed in the same manner as adults, with a diagnostic
acetaminophen level drawn at 4 hours post ingestion or as soon as
possible thereafter. The dose of acetylcysteine is the same on a weight
basis. However, in children who weigh less that 40 kg, the administration
of acetylcysteine by the intravenous route should be altered because the
dilution provides excessive free water and may result in symptomatic
hyponatremia. Consult the Acetadote
®
package insert for information on
handling these cases*.
b. Pregnant women
The use of acetylcysteine does not change for pregnant patients. (See Deter-
mining the Need for Acetylcysteine and Administration of Acetylcysteine)
c. Patients presenting 24 hours or more postingestion
An acetaminophen level and the serum ALT or AST concentration should
be determined in patients presenting 24 hours or more postingestion. No
further treatment is needed for patients without liver injury (ALT and AST
levels are normal) if the acetaminophen level is also <10 mcg/mL.
In patients with an increased serum ALT and/or AST, treatment with
acetylcysteine should be initiated. Evidence suggests that acetylcysteine
treatment may improve survival in patients presenting late and may be
appropriate almost any time after overdose ingestion. A controlled study
reported that intravenous acetylcysteine improves survival in patients
with established fulminant hepatic failure, caused by purposeful overdose
of acetaminophen, who presented 36 to 80 hours postingestion*.
d. Chronic alcohol users
Chronic heavy alcohol users may be at an increased risk for hepatic
injury and death following excessive acetaminophen use, although
reports of this event are rare. In these cases, acetylcysteine treatment is
recommended using the same indications for treatment and method of
administration as described for other patients. (See Determining the Need
for Acetylcysteine and Administration of Acetylcysteine)
e. Obese patients
The standard recommended doses of acetylcysteine are weight based for
both the oral and intravenous protocols. There are no controlled data eval-
uating the safety and necessity of the high doses of acetylcysteine that
would be given to obese patients. Although no data exist, the manufac-
turer of the intravenous acetylcysteine product recommends a maximum
dose based on 100 kg (15 gm loading dose followed by 5 g over 4 hours
followed by 62.5 mg/hr) for subjects who weigh more than 100 kg.
f. Other diseases
Several drug-disease interactions have been postulated for acet-
aminophen. These conditions include infectious hepatitis, alcoholism,
malnourishment, treatment with medications known to induce cyto-
chrome 2E1 (CYP2E1), acquired immunodeficiency syndrome (AIDS),
starvation, and liver injury from another cause in the presence of acet-
aminophen use. No prospective data have supported concerns about
using labeled doses of acetaminophen in these patients or addressed the
management of these patients following an acetaminophen overdose.
There is no evidence that these patients would benefit from a different
risk assessment strategy or management approach, although some
toxicologists will treat acetaminophen overdoses that occur in certain
populations or in patients taking medication known to induce CYP2E1
(such as isoniazid or rifampin) at a lower threshold. It is recommended
that these patient groups be treated in the same manner as other patients
with acetaminophen overdose.
MANAGEMENT OF ACUTE OVERDOSE
* This monograph is intended to assist practitioners in managing acetaminophen overdoses and is not meant as a standard of care. For further information
and individualized consultation concerning complex or difficult cases, please contact your local poison center (1-800-222-1222) or a clinical toxicologist.
McNeil Consumer Healthcare sponsors a toll-free telephone number (1-800-525-6115), available 24 hours a day, at the Rocky Mountain Poison and Drug Center.

(acetaminophen)
47
For patients 6 years or older, RSTI is defined
as ingestion of 1) at least 10g or 200 mg/kg
(whichever is less) over a single 24 hour period,
or b) at least 6 g or 150 mg/kg (whichever is
less) per 24 hour period for the preceding 48
hours or longer. For patients younger than 6
years of age, RSTI is defined as ingestion of
a) 200 mg/kg or more over a single 24 hour
period, or b) 150 mg/kg or more per 24 hour
period for the preceding 48 hours, or c) 100
mg/kg or more per 24 hour period for the
preceding 72 hours or longer. The Rumack-Mat-
thew nomogram cannot be used in these cases.
A number of patients have experienced liver
injury following repeated supratherapeutic
ingestion of acetaminophen. Most of these
patients ingested large doses over a period
of several days.
Management
of Repeated Chronic
Supratherapeutic Ingestion
Patients who report a history of RSTI should
have the acetaminophen level and transaminase
activity determined. If the acetaminophen level
is <20 mcg/mL AND the ALT or AST is normal,
no treatment is indicated however the patient
should be instructed on appropriate acetamin-
ophen use. If either the ALT or AST is elevated
OR the acetaminophen level is >20 mcg/mL,
acetylcysteine is indicated.
The duration of acetylcysteine treatment has
not been established for RSTI. A common
approach for the treatment of RSTI is to treat
the patient with acetylcysteine for 12 hours and
then reevaluate. If the patient is clinically well,
the ALT and AST are improving, and the acet-
aminophen level is <10 mcg/mL, acetylcysteine
may be discontinued. Chronic users of alcohol
are assessed and treated for RSTI in the same
manner as other patients. A stepwise guide for
managing repeated supratherapeutic acetamin-
ophen overdose is provided in Flowchart 2*.
* This monograph is intended to assist practitioners in managing acetaminophen overdoses and is not meant as a standard of care. For further information
and individualized consultation concerning complex or difficult cases, please contact your local poison center (1-800-222-1222) or a clinical toxicologist.
McNeil Consumer Healthcare sponsors a toll-free telephone number (1-800-525-6115), available 24 hours a day, at the Rocky Mountain Poison and Drug Center.

(acetaminophen)
48
The principal toxic effect of a substantial acetaminophen overdose is hepatic injury. Normally,
acetaminophen metabolism involves 3 separate pathways: (1) conjugation with glucuronide
(glucuronidation); (2) conjugation with sulfate (sulfation); and (3) metabolism via the cytochrome
P450-dependent mixed function oxidative enzyme pathway to form a reactive intermediate metab-
olite. The reactive intermediate metabolite formed through the P450 pathway conjugates with
glutathione and is then further metabolized to form cysteine and mercapturic acid conjugates. The
acetaminophen glucuronide, acetaminophen sulfate, and glutathione derived metabolites are not
toxic. Thus, toxicity does not occur with normal therapeutic use.
Following a substantial overdose, however, the amount of reactive intermediate metabolite
produced may increase markedly. The amount of glutathione available in the liver may become
insufficient to conjugate and detoxify the reactive intermediate metabolite. It is estimated that
when the amount of available glutathione is reduced to approximately 30% of normal, the reactive
intermediate metabolite binds to hepatic cell macromolecules, producing cellular necrosis. The
exact mechanism of hepatocellular damage is not known, but is reflected by a rise in serum trans-
aminases. With increasing hepatocellular necrosis, hepatic dysfunction occurs. In severe cases, this
may proceed to hepatic failure.
Signs and symptoms of acetaminophen overdose, during the initial management phase, show a
typical pattern but are not diagnostic or predictive of risk. The clinical course of acetaminophen
overdose generally occurs in a 3-phase sequential pattern:
Clinical Characteristics
of Acute Acetaminophen Overdose
Phase I
The first phase begins shortly after ingestion of a potentially toxic overdose and lasts for 12 to 24
hours. The patient may manifest signs of gastrointestinal irritability, nausea, vomiting, anorexia,
diaphoresis, and pallor. The larger the overdose, the more likely it is that these symptoms are pres-
ent. Coma or other evidence of central nervous system depression is usually not present unless the
patient has taken a massive overdose or has also ingested central nervous system depressants, as
may be the case in suicide attempts. Coma accompanied by severe metabolic acidosis has rarely
been reported following acetaminophen overdose, but the loss of consciousness was thought to
be secondary to the metabolic acidosis rather than the acetaminophen itself. In small children,
spontaneous vomiting following a substantial overdose occurs frequently and may play a role in the
reduced risk of toxicity in children. However, these symptoms are not unique to acetaminophen,
and unless the possibility of acetaminophen overdose is considered during this early phase, it may
be overlooked. Many patients with early symptoms never progress beyond the first phase and
recover without additional problems.
Phase II
If toxicity continues or is to ensue, there is a latent phase in terms of clinical findings of up to
48 hours. Initial symptoms abate and the patient may feel better. However, hepatic enzymes,
bilirubin, lactate, phosphate, and prothrombin time or INR values will progressively rise, with
hepatic enzymes often rising to striking levels. Right upper quadrant pain may develop as the liver
becomes enlarged and tender. Most patients do not progress beyond this phase, especially if given
acetylcysteine treatment. The subsequent clinical course is characterized by a gradual return of
liver enzyme tests to normal.
Phase III
A few patients will develop serious hepatic
necrosis. Signs and symptoms of this third
phase of the clinical course depend on the
severity of hepatic damage and usually occur
from 3 to 5 days following ingestion. The peak
AST and ALT occurs between 72 to 96 hours
post ingestion. Symptoms may be limited to
anorexia, nausea, general malaise, and abdom-
inal pain in less severe cases or may progress
to confusion, stupor, and sequelae of hepatic
necrosis including jaundice, coagulation
defects, hypoglycemia, and encephalopathy,
as well as renal failure and cardiomyopa-
thy. Death, if it occurs, is generally a result
of complications associated with fulminant
hepatic failure. Mortality rates in patients with
toxic acetaminophen levels who do not receive
antidotal therapy are in the range of 3% to 4%.
In nonfatal cases, serial liver biopsies and liver
enzyme tests have shown prompt resolution
without significant residual functional or archi-
tectural alterations of the liver.

(acetaminophen)
49
Acetaminophen overdose can be effectively managed
by focusing on a few basic principles. As in all
cases of poisoning, healthcare providers should
obtain a careful history and should have a high
index of suspicion. When acetaminophen overdose
is a possibility, an acetaminophen level should be
obtained and antidotal therapy should be initiated as
indicated in these guidelines. When acetylcysteine
is administered soon after an overdose occurs,
morbidity is significantly reduced and mortality
virtually eliminated. The prognosis for patients with
acetaminophen overdose is excellent, provided
treatment is given expeditiously and appropriately.
Summary

(acetaminophen)
50
Flowchart 1. Stepwise Management of Acute Acetaminophen Overdose
Estimate time of ingestion
< 24h since overdose
Administer activated charcoal
if < 2h post ingestion
Serum level plots
BELOW treatment line
Stop acetylcysteine
Initiate (or continue)
acetylcysteine
Obtain baseline tests
(ALT and AST
chemistries) and
provide supportive
care as indicated
Serum level plots ABOVE
treatment line
AA
Determine acetaminophen level
at 4h post ingestion*, or as
soon as possible thereafter**
PLOT ON NOMOGRAM
Consider starting
acetylcysteine treatment
if acetaminophen level will
not be available before 8
hours after ingestion
A
> 24h since overdose
Start acetylcysteine and
manage in accordance with
serum ALT and AST
(see Flowchart 2)
*Acetaminophen levels obtained before 4
hours after ingestion should not be used
for risk stratification
**With extended-release preparations, serum
acetaminophen levels drawn less than 8 hours
post-ingestion may not represent peak levels.
It may be prudent to obtain a second level 4
to 6 hours after the initial level was drawn
A
Acetylcysteine can be withheld until
actaminophen assay results are available as
long as initiation of treatment is not delayed
beyond 8 hours post-ingestion. If more than 8
hours post-ingestion, acetylcysteine
treatment must be started immediately.
AA
With the extended-release preparation,
provide acetylcysteine treatment if either
level plots above the treatment line.
ALT=alanine transaminase; AST=aspartate
transaminase

(acetaminophen)
51
Flowchart 2. Stepwise Management of Repeated Supratherapeutic Ingestion
Draw serum ALT and AST
and acetaminophen level
History of repeated supretherapeutic ingestion
No further treatment needed
ALT = alanine transaminase; AST = aspartate transaminase
ALT of AST >50 IU/L
OR
Acetaminophen >20 mcg/mL
ALT and AST <50 IU/L
AND
Acetaminophen <20 mcg/mL
Treat with acetylcysteine for 12
hours and reevaluate.
Acetylcysteine may be
discontinued if patient is
clinically well, ALT and AST are
improving, and acetaminophen
level is <10 mcg/mL.
(acetaminophen)
52
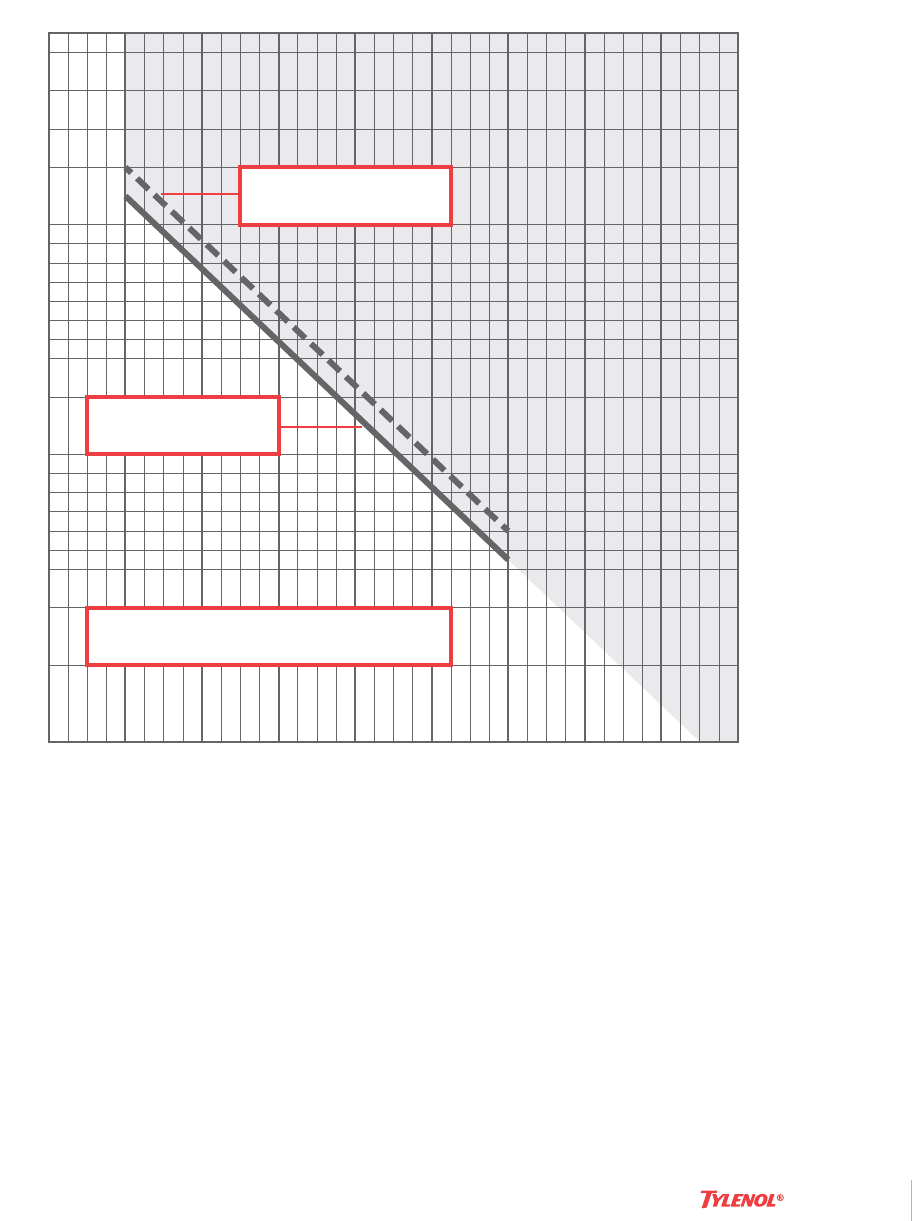
Chart 1. Rumack-Matthew Nomogram
Nomogram: Acetaminophen plasma concentration versus time after
acetaminophen ingestion (adapted with permission from Rumack and
Matthew, Pediatrics. 1975;55:871-876). The nomogram has been devel-
oped to estimate the probability of whether an acetaminophen level in
relation to the interval postingestion will result in hepatotoxicity and,
therefore, whether acetylcysteine therapy should be administered. Values
above the Rumack-Matthew line connecting 200 mcg/mL at 4 hours with
50 mcg/mL at 12 hours are reported to be associated with a potentially
increased risk of hepatotoxicity if acetylcysteine is not administered. In
order to err on the side of safety, a treatment line has been established
that is 25% below the Rumack-Matthew line.
Cautions For Use Of This Chart:
1. Time coordinates refer to time postingestion.
2. Graph relates only to plasma (or serum) concentrations following a
single, acute overdose ingestion.
3. The Treatment Line is plotted 25% below the Rumack-Matthew Line
to allow for potential errors in acetaminophen assays and estimated
time from ingestion of an overdose (Rumack et al. Arch Intern Med
1981;141(suppl):380-385).
4
2
3
4
812162024283236
5
6
7
8
9
10
20
30
40
50
60
70
80
90
100
300
400
500
200
150
Treatment Line
Rumack-Matthew Line
Treatment should be administered
if level is above solid line
μg/ml
Acetaminophen Plasma Concentration (mcg/mL)
Hours Postingestion

(acetaminophen)
53
Chart 2. Common Adverse Events Associated with the Oral and Intravenous
Formulations of N-acetylcysteine.
Intravenous
Gastrointestinal Anaphylactoid Other
Nausea
Vomiting
Bronchospasm
Pruritus
Flushing
Urticaria
Nonurticarial rash
Chest tightness
Hypotension
Tachycardia
Presyncope/syncope
Anxiety
Oral
Gastrointestinal Anaphylactoid Other
Nausea
Vomiting
Bronchospasm
Pruritus
Hypotension
Tachycardia

(acetaminophen)
54
ACETADOTE (acetylcysteine) injection, solution. Cumberland Pharmaceu-
ticals Inc.; Nashville, Tennessee; http://dailymed.nlm.nih.gov/dailymed/
lookup.cfm?setid=472f158a-5ab9-4308-8e49-1116e6ea3d39.
Betten DP, Cantrell FL, Thomas SC, Williams SR, Clark RF. A prospective
evaluation of shortened course oral N-acetylcysteine for the treatment of
acute acetaminophen poisoning. Ann Emerg Med. 2007;50:272-279
Bray GP, Harrison PM, O’Grady JG, Tredger JM, Williams R. Long-term
anticonvulsant therapy worsens outcome in paracetamol-induced fulmi-
nant hepatic failure. Hum Exp Toxicol. 1992;11:265-270.
Bond GR. Reduced toxicity of acetaminophen in children: It’s the liver.
J Toxicol Clin Toxicol. 2004;42:149-152.
Bond GR: A new acetaminophen nomogram with at different purpose
(editorial). Ann Emerg Med. 2005:46;272-274.
Buckley NA, Whyte IM, O’Connell DL, Dawson AH. Oral or intravenous
N-acetylcysteine: which is the treatment of choice for acetaminophen
(paracetamol) poisoning? J Toxicol Clin Toxicol. 1999;37:759-767.
Cetaruk EW, Dart RC, Hurlbut KM, Horowitz RS, Shih R. Tylenol Extended
Relief overdose. Ann Emerg Med. 1997;30:104-108.
Curry SC, Braitberg G. Poisoning in pregnancy. In: Foley MR, Strong TH Jr,
eds. Obstetric Intensive Care. Philadelphia, PA: W.B. Saunders Company;
1997:347-367.
Daly FF, O’Malley GF, Heard K, Bogdan GM, Dart RC. Prospective eval-
uation of repeated supratherapeutic acetaminophen (paracetamol)
ingestion. Ann Emerg Med. 2004;44:393-398.
Dart RC, Erdman AR, Olson KR, Christianson G, Manoguerra AS, Chyka
PA, Caravati EM, Wax PM, Keyes DC, Woolf AD, Scharman EJ, Booze
LL, Troutman WG; American Association of Poison Control Centers.
Acetaminophen poisoning: an evidence-based consensus guideline for
out-of-hospital management. Clin Toxicol. 2006;44:1-18.
Doyon S, Klein-Schwartz W. Hepatotoxicity despite early administration
of intravenous N-acetylcysteine for acute acetaminophen overdose. Acad
Emerg Med. 2009;16(1):34-9.
Duggan ST, Scott LJ. Intravenous Paracetamol (Acetaminophen). Drugs.
2009; 69:101-113.
Gray T, Hoffman RS, Bateman DN. Intravenous paracetaml-an interna-
tional perspective of toxicity. Clin Toxicol. 2011;49:150-2.
Acetaminophen Overdose:
Suggested Readings
Harrison PM, Keays R, Bray GP, Alexander GJM, Williams R. Improved
outcome of paracetamol-induced fulminant hepatic failure by late admin-
istration of acetylcysteine. Lancet. 1990;335:1572-1573.
Heard K, Green JL, Bailey JE, Bogdan GM, Dart RC. A randomized trial
to determine the change in alanine aminotransferase during 10 days of
paracetamol (acetaminophen) administration in subjects who consume
moderate amounts of alcohol. Aliment Pharmacol Ther. 2007;26:283-290.
Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med.
2008;359:285-292.
Horowitz RS, Dart RC, Jarvie DR, Bearer CF, Gupta U. Placental transfer
of N-acetylcysteine following human maternal acetaminophen toxicity. J
Toxicol Clin Toxicol. 1997;35:447-451.
Keays R, Harrison PM, Wendon JA, et al. Intravenous acetylcysteine in
paracetamol induced fulminant hepatic failure: a prospective controlled
trial. Br Med J. 1991;303:1026-1029.
Kerr F, Dawson A, Whyte IM, Buckley N, Murray L, Graudins A, Chan
B, Trudinger B. The Australasian Clinical Toxicology Investigators
Collaboration randomized trial of different loading infusion rates of N-ace-
tylcysteine. Ann Emerg Med. 2005;45:402-408.
Khandelwal N, James LP, Sanders C, Larson AM, Lee WM and the Acute
Liver Failure Study Group. Unrecognized acetaminophen toxicity as a
cause of indeterminate acute liver failure. Hepatology. 2011;53:567-576.
Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch
JS, Shiodt FV, Ostapowicz G, Shakil AO, Lee WM and the Acute Liver Fail-
ure Study Group. Acetaminophen-induced liver failure: results of United
States multicenter, prospective study. Hepatology. 2005; 42:1364-72.
Mitchell JR, Thorgeirsson SS, Potter WZ, Jollow DJ, Keiser H. Acetamin-
ophen-induced hepatic injury: protective role of glutathione in man and
rationale for therapy. Clin Pharmacol Ther. 1974;16:676-684.
Ofirmev (acetaminophen) injection, solution. Cadence Pharmaceuti-
cals, Inc; San Diego, CA; http://dailymed.nlm.nih.gov/dailymed/lookup.
cfm?setid=c5177abd-9465-40d8-861d-3904496d82b7
Polson J, Wians FH, Orsulak P, Fuller D, Murray NG, Koff JM, Khan AI,
Balko JA, Hynan LS, Lee WM, and the Acute Liver Failure Study Group.
False Positive acetaminophen concentrations in patients with liver injury.
Clin Chim Acta. 2008;391:24-30..

(acetaminophen)
55
Roth B, Woo O, Blanc P. Early metabolic acidosis and coma after acet-
aminophen ingestion. Ann Emerg Med. 1999;33:452-456.
Rumack BH. Acetaminophen hepatotoxicity: the first 35 years. J Toxicol
Clin Toxicol. 2002;40:3-20.
Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediat-
rics. 1975;55:871-876.
Rumack BH, Peterson RC, Koch GG, Amara IA. Acetaminophen overdose:
662 cases with evaluation of oral acetylcysteine treatment. Arch Intern
Med. 1981;141(suppl):380-385.
Sivilotti M, Yarema MC, Juurlink DN, Good AM, Johnson DW. A risk quan-
tification instrument for acute acetaminophen overdose patients treated
with N-acetylcysteine. Ann Emerg Med. 2005;46:263-271.
Smilkstein MJ, Bronstein AC, Linden C, Augenstein WL, Kulig KW,
Rumack BH. Acetaminophen overdose: A 48-hour intravenous N-acetyl-
cysteine treatment protocol. Ann Emerg Med. 1991;20:1058-1063.
Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-ace-
tylcysteine in the treatment of acetaminophen overdose: analysis of the
National Multicenter Study (1976 to 1985). N Engl J Med. 1988;319:1557-1562.
Sztajnkrycer MD, Bond GR. Chronic acetaminophen poisoning in children.
Curr Opin Pediatr. 2001;13:177-182.
Whyte IM, Buckley NA, Reith DM, Goodhew I, Seldon M, Dawson AH.
Acetaminophen causes an increased International Normalized Ratio by
reducing functional factor VII. Ther Drug Monit. 2000;22:742-8.
Yarema MC, Johnson DW, Berlin RJ, Sivilotti ML, Nettel-Aquirre A, Brant
RF, Spyker DA, Bailey B, Chalut D, Lee JS, Plint AC, Purssell RA, Rutledge
T, Sevior CA, Stiell IG, Thompson M, Tyberg J, Dart RC, Rumack BH.
Comparison of the 20-hour intravenous and 72-hour oral acetylcyste-
ine protocols for the treatment of acute acetaminophen poisoning. Ann
Emerg Med. 2009;54:606-14.
ACETAMINOPHEN OVERDOSE: SUGGESTED READINGS
